Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (38): 5745-5751.doi: 10.3969/j.issn.2095-4344.2016.38.018
Previous Articles Next Articles
Collagen/silk fibroin nerve conduits used for repairing peripheral nerve defect: application and development
- 1Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin 300052, China; 2Institute of Medical Equipment, Academy of Military Medical Sciences, Tianjin 300161, China
-
Received:2016-07-06Online:2016-09-16Published:2016-09-16 -
Contact:Xu Yun-qiang, Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin 300052, China -
About author:Xu Yun-qiang, M.D., Associate chief physician, Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin 300052, China -
Supported by:the National Natural Science Foundation of China, No. 81101362
CLC Number:
Cite this article
Xu Yun-qiang, Liu Ying-jie, Li Rui-xin, Zhu Shuang-long, Zhang Zhen-hui.
share this article
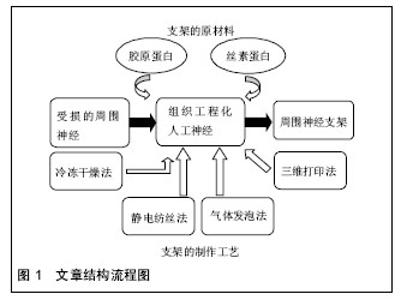
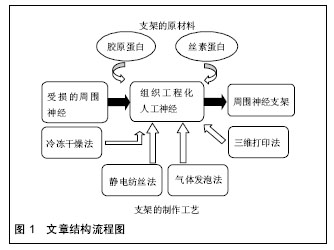
2.1 纳入资料基本概况 纳入的文献包括周围神经缺损概述12篇[1-12],探讨胶原蛋白及丝素蛋白构建神经支架的优缺点19篇[13-31],探讨神经支架构建的制作工艺17篇[32-46]。以此为依据对用于构建神经支架材料的选择和支架构建的工艺对神经再生的影响进行了归纳和总结。见图1。 2.2 纳入资料的研究结果特征 2.2.1 神经支架的材料 胶原蛋白:是哺乳动物中普遍存在的大分子蛋白质,大部分由哺乳动物中的结缔组织分泌,小部分来自肝脏、肺脏、脾脏和骨骼等器官分泌,是细胞外基质的主要组成部分,其自身结构与一般蛋白质不同,它是由多条具有左手螺旋结构的多肽链构成,各自以氢键相互交合,最终构成右手超螺旋[13]。已有研究证实,胶原能被分布在细胞表面的整合素识别并结合[14],孔隙联通的胶原蛋白支架可以促进血管的新生[15]。以胶原为支架材料植入周围神经损伤部位,神经细胞能够存活生长,并有助于神经轴突的延伸[15-16]。近来研究证实,将血管内皮细胞生长因子或肝素结合于胶原蛋白支架上,比单纯应用胶原支架更有效诱导血管的新生[17]。Okamoto等[18]选用胶原蛋白作为构建神经导管的原材料,修复有30 mm长犬的坐骨神经缺损,通过实验观察,导管与宿主的相容性很好,神经功能恢复良好。有研究对Ⅰ/Ⅲ型胶原复合导管的生物相容性进行了研究发现,术后5-7 d导管与宿主得到很好的结合[19]。Lu等[20]通过对胶原蛋白材料性能的研究,发现胶原蛋白用于支架制备,缺乏一定的力学性能,难于塑形,且在生物体内降解过快。胶原凝胶、功能化胶原凝胶均具有优良的生物相容性[21-22]。但是,采用单一胶原构造三维多孔神经支架,结构不稳定,同时具有生物机械性能差和体内降解速度快等缺点。要克服胶原蛋白在神经修复过程中的缺点,除了对胶原蛋白自身改性加工外,同时还需开发特性互补的天然生物材料。 丝素蛋白:是从蚕丝中提取的高分子生物材料,由丙氨酸、丝氨酸和酪氨酸等18种氨基酸通过S-S键相互结合L链和H链构成,因为丝素蛋白主要成分中含有大量氨基,极易形成具有氢键结构的β折叠结晶,因而丝素蛋白不溶于水[23]。但是,它在碱性溶液中,β折叠结晶易被破坏进而分解,降低了力学性能。丝素蛋白具有较好的透气和透湿性,对氧和水的通透性较高,可被自身蛋白酶降解成寡肽或氨基酸[24]。丝素具有较好的柔韧性,而在材料自身的物理性能上与自体神经十分接近[25]。Yang等[26]利用丝素蛋白制备周围神经微导管修复坐骨神经缺损实验证明,大鼠的坐骨神经缺损处愈合良好,促进神经的再生及损伤轴突的延伸;同时也表明了丝素蛋白作为导管材料能被生物降解吸收,但其降解速率缓慢。Tang等[27]研究发现,把丝素蛋白制作成神经微导管后,联合许旺细胞、背根神经节细胞和促生长因子共培养4周,再将神经微导管植入约有10 mm缺损的大鼠坐骨神经中,12周后进行神经组织学及功能评价,发现丝素蛋白神经微导管组显著改善了神经轴突的延伸,同时增高了PMP22及神经钙黏素的表达,有助于缺损坐骨神经的再生。黄训亭等[28]通过对丝素蛋白多孔膜生物降解性能的研究,发现丝素蛋白植入体内后,可以被某些蛋白酶降解,但在体内降解缓慢,其降解速率很难与神经再生速度相匹配,达不到很好的修复效果。 综上,丝素蛋白在组织工程神经研究中,作为支架制备的原材料,一方面具有生物相容性好和与自身神经相似的力学性能等优点,另一方面又存在体内降解缓慢等不足,单一丝素蛋白作为修复神经损伤不能更好的构建利于缺损神经修复的微环境,所以还需开发通过联合其他生物材料弥补自身构建神经导管的缺点。 胶原/丝素蛋白:将胶原蛋白和丝素蛋白按一定比例复合,即可在保持良好细胞相容性的基础上,提高单一胶原蛋白的力学性能;与此同时,也改善了丝素蛋白在体内降解缓慢的不足,可以为受损神经提供利于细胞生长的微环境。胶原/丝素蛋白复合,一方面改善了单一胶原蛋白膜的抗水性能,同时降低其热水溶失率[29]。Kim等[30]把丝素蛋白与胶原蛋白联合后,发现材料自身的表面形状发生改变,而与单一天然原材料相比,复合材料表面与酶溶液的接触面积大大增加,从而改良了单一丝素蛋白神经导管降解缓慢的问题,同时也改善了单一胶原蛋白材料机械性能的缺陷,更利于周围神经修复再生。Xu等[31]选用胶原蛋白和丝素蛋白作为原材料构建神经导管修复缺损的坐骨神经,研究证实了,胶原/丝素蛋白导管对坐骨神经缺损的修复,具有良好的促神经生长和桥接作用。因此,胶原/丝素蛋白复合材料有望成为未来修复周围神经损伤的理想人工微导管材料。 2.2.2 神经支架制作的工艺 冷冻干燥法:基本原理是将高分子材料(如丝素蛋白或胶原蛋白)制成凝胶状,放入具有较低压强及温度的真空冷冻干燥机中,进行快速冷冻,样品发生汽化进而与高分子材料分离,样品中结晶的水升华,继而在原位置上形成多样孔隙,最后得到不同尺寸、孔隙连通率较高的聚合物。目前,该方法在组织工程支架制备上得到了广泛的应用,主要通过调节反应的温度、浓度和时间来调控支架微孔的形状和尺寸。Mandal等[32]采用冷冻干燥法构建的丝素蛋白支架,主要通过调节冷冻温度进而改变支架的微孔性能,研究发现,随着温度的调低,支架的孔径变小,但其孔隙率升高。Bhardwaj等[33]选用丝素为原材料,再联合冷冻干燥法制备三维支架,研究发现,通过该法制备的丝素蛋白支架,微孔分布更均衡、孔隙率高达90%,且微孔间的连通性也好,抗压应力高,有利于细胞的增殖及分化。 冷冻干燥法可在低温条件下操作,制备出的神经支架具有良好的细胞相容性,且微孔间连通率高,还可以通过调节反应条件制造出不同孔径的神经支架;但是,该法制备的支架孔径一般偏小,且容易形成片状不规则结构。 静电纺丝法:是目前在人工神经支架制备上迅速发展的一种简单有效的新兴制作工艺。静电纺丝法先将天然高分子材料溶解于有机溶剂中形成复合共混液,再将共混液倒入静电纺丝装置中,利用电场力使喷洒装置上喷口处的液滴表面凝聚电荷,使聚合物液体带上几千伏甚至上万伏的高压静电。随着电场逐渐升高时,针头处的液滴会由球形逐渐拉长变为圆锥形,形成“泰勒锥”。随着电场强度升高达到临界值时,电场力就能克服液滴的表面张力,进而从“泰勒锥”中射出,形成快速拉伸、变形的聚合物微小射流,同时溶剂也迅速挥发最终固化成纳米级聚合纤维。Jun等[34]将聚己内酯和丝素蛋白按一定比例混溶在甲酸溶液中,采用静电纺丝技术制备支架,结果表明,共混改善了单一材料的降解性及细胞相容性。Kemp等[35]应用胶原和硅胶共溶液作为原材料,联合静电纺丝技术制备修复周围神经损伤的导管支架,研究表明,共混组微血管再生较快,促进了轴突的延伸,更利于损伤神经的修复和功能恢复。Schnell等[36]将胶原和PCL以25%∶75%的比例共混于有机溶剂中,制成混合溶液后,再采用静电纺丝法制备胶原-PCL纳米纤维支架,研究发现许旺细胞在该复合纤维支架上的黏附、增殖、分化等生物学行为均比单纯应用PCL或胶原支架好。静电纺丝法制备的神经导管支架,最大程度模仿了天然细胞外基质,为周围神经细胞修复再生提供合适生长微环境,还可以对导管支架表面修饰加工,譬如,给支架结合并缓释药物和核酸等物质,从而达到通过调控生物化学信号的变化来控制细胞的生物学行为,这样更利于细胞的增殖和分化[37-38]。但是,如果要制成三维神经支架,则存在孔径大小和微孔分布很难控制等问题。 气体发泡法:一般包括化学和物理两种发泡方式。其中化学发泡法在神经支架的制备上较常用,化学发泡法一般选用碳酸盐类化合物作为发泡剂,即将支架原材料溶液和碳酸盐类化合物按一定比例混合后加入到模具中,然后再蒸发掉溶剂,并用热水浴或真空干燥,真空干燥引起碳酸盐溶剂升华,热水浴使气体挥发,最后得到多孔支架。Nazarov等[39]将丝素蛋白和六氟异丙醇按一定比例配制成一定浓度的混合溶液后,将其倒入装有NH4HCO3颗粒的模具中,室温条件下,让溶液挥发,随后将导管支架进行热水浴发泡,直至没有气泡形成,而后进行常温水浴再干燥,最终得到多孔支架。研究证实得到的支架孔隙率达90%以上,微孔间连通性良好,抗压强度高,而且支架表面没有留下厚厚的发生层,符合组织工程支架要求。气体发泡法制得的神经支架孔隙率可达90%且均匀,有效避免了有机溶剂对神经支架活性的影响,也减少了表皮层的产生,但很难控制支架中孔隙分布且操作较复杂。 致孔剂制孔法:是把致孔剂颗粒放入生物材料溶液中,搅拌使两者混匀,而后固化,其中利用两者具有不同的溶解性,析出致孔剂,最后干燥得到多孔生物材料支架,支架的微孔是由致孔剂所致。常采用的致孔剂有氯化钠颗粒[40]及石蜡颗粒[41]等。Bhardwaj等[42]选用氯化钠颗粒作为致孔剂制备的丝素蛋白冻干支架,结果证实经制孔剂制得的支架具有良好的机械性能和孔隙率,还能促进细胞的增殖和分化,证明致孔剂制孔法在组织工程制备三维支架领域上具有广阔的应用前景。致孔剂制孔法可以通过选用不同的致孔剂颗粒和加入量来调节神经支架中的孔径和孔隙率,但很难控制孔隙的分布,同时也存在连通性不好。 三维打印法:是利用计算机辅助设计支架模型、联合数控技术、计算机断层扫描、MRI影像等技术为一体数据重建支架模型,得出三维支架模型,再通过快速成型打印机进行三维打印,采用对不同高分子材料的精准分层堆积,制作出预期复杂形状的三维神经支架的数字化快速成型技术[43-44],因此也被称为快速三维成型技术。因为周围神经细胞外基质具有如凹槽、隆起等特性,其不仅使细胞生长形态呈双极状,同时也能引导细胞定向迁移[45]。因此,设计出与体内细胞微环境相似的三维结构支架,这样更利于细胞存活、增殖、迁移和分化[46]。 目前,应用于组织工程支架制作领域的快速成型技术主要有激光固化技术、高温熔融沉积技术和三维打印技术,其中激光固化成形技术需要光敏材料,固化后易产生收缩和扭曲,而高温熔融沉积技术的高温环境对生物材料的性能,尤其是生物活性有不可避免的影响。而低温快速成形技术可以有效解决上述工艺存在的局限性,此技术是将液态或胶冻状材料均匀混合之后,根据设计的模型通过三维打印设备制成三维多孔结构支架,冷冻干燥后溶剂升华,留下大量微孔。同时,三维打印法具有可精准控制支架的孔径及其分布,可打印各种形状的支架,耗时短,能够进行大批量生产等优点。该方法具有精确、快速等优势,其将在人工神经制备领域中得到更广泛的应用。 "
| [1]Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521-534. [2]Eser F, Aktekin L A, Bodur H, et al. Etiological factors of traumatic peripheral nerve injuries. Neurol India. 2009;57(4):434-437. [3]Han WJ, Zhang XF, Yang SM, et al. Surgical management and prognosis of iatrogenic peripheral facial nerve injury following middle ear surgery. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46(12):998-1004. [4]张勇杰,金岩,聂鑫,等.组织工程周围神经修复坐骨神经缺损应用研究[J].中华神经外科疾病研究杂志, 2004,3(2): 141-144. [5]张彩顺,吕刚.无细胞神经支架复合骨髓间充质干细胞构建组织工程人工神经修复坐骨神经缺损[J].中国组织工程研究与临床康复,2011,15(25):4591-4596. [6]张文捷,周跃,王建忠,等.应用异体许旺细胞构建组织工程人工神经复合体的体外实验[J].中国临床康复, 2005, 9(14): 46-47. [7]Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98(1):16-37. [8]Valek L, Kanngiesser M, Haussler A, et al. Redoxins in peripheral neurons after sciatic nerve injury. Free Radic Biol Med. 2015;89:581-592. [9]Ruijs AC, Jaquet JB, Kalmijn S, et al. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116(2): 484-496. [10]IJkema-Paassen J, Jansen K, Gramsbergen A, et al. Transection of peripheral nerves, bridging strategies and effect evaluation. Biomaterials. 2004;25(9): 1583-1592. [11]Elkwood AI, Holland NR, Arbes SM, et al. Nerve allograft transplantation for functional restoration of the upper extremity: case series. J Spinal Cord Med. 2011; 34(2):241-247. [12]Gu X, Ding F, Yang Y, et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011; 93(2):204-230. [13]Anguiano M, Castilla C, Maska M, et al. Characterization of the role of collagen network structure and composition in cancer cell migration. Conf Proc IEEE Eng Med Biol Soc. 2015;2015: 8139-8142. [14]White DJ, Puranen S, Johnson MS, et al. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36(8):1405-1410. [15]Callegari A, Bollini S, Iop L, et al. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials. 2007; 28(36):5449-5461. [16]Pearson J, Ganio MS, Schlader ZJ, et al. Post junctional sudomotor and cutaneous vascular responses in noninjured skin following heat acclimation in burn survivors. J Burn Care Res. 2016. [17]Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4(3):477-489. [18]Okamoto H, Hata K, Kagami H, et al. Recovery process of sciatic nerve defect with novel bioabsorbable collagen tubes packed with collagen filaments in dogs. J Biomed Mater Res A. 2010;92(3): 859-868. [19]Keilhoff G, Stang F, Wolf G, et al. Bio-compatibility of type I/III collagen matrix for peripheral nerve reconstruction. Biomaterials. 2003;24(16):2779-2787. [20]Lu Q, Feng Q, Hu K, et al. Preparation of three-dimensional fibroin/collagen scaffolds in various pH conditions. J Mater Sci Mater Med. 2008;19(2):629-634. [21]Goto E, Mukozawa M, Mori H, et al. A rolled sheet of collagen gel with cultured Schwann cells: model of nerve conduit to enhance neurite growth. J Biosci Bioeng. 2010;109(5):512-518. [22]Keilhoff G, Stang F, Wolf G, et al. Bio-compatibility of type I/III collagen matrix for peripheral nerve reconstruction. Biomaterials. 2003;24(16):2779-2787. [23]Jin Y, Hang Y, Luo J, et al. In vitro studies on the structure and properties of silk fibroin aqueous solutions in silkworm. Int J Biol Macromol. 2013;62: 162-166. [24]Zhang X, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials. 2008;29(14):2217-2227. [25]吴刚,董长超,王光林,等.PLGA-丝素-胶原纳米三维多孔支架材料的制备及细胞相容性研究[J].中国修复重建外科杂志,2009,23(8):1007-1011. [26]Catto V, Fare S, Cattaneo I, et al. Small diameter electrospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater Sci Eng C Mater Biol Appl. 2015;54:101-111. [27]Cui F, Li J, Ding A, et al. Conditional QTL mapping for plant height with respect to the length of the spike and internode in two mapping populations of wheat. Theor Appl Genet. 2011;122(8):1517-1536. [28]黄训亭,邵正中,陈新.天然蚕丝与丝素蛋白多孔膜的生物降解性研究[J].化学学报, 2007,65(22):2592-2596. [29]Levin B, Redmond SL, Rajkhowa R, et al. Utilising silk fibroin membranes as scaffolds for the growth of tympanic membrane keratinocytes, and application to myringoplasty surgery. J Laryngol Otol. 2013;127 Suppl 1:S13-S20. [30]Kim UJ, Park J, Kim HJ, et al. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775-2785. [31]Xu Y, Zhang Z, Chen X, et al. A Silk Fibroin/Collagen Nerve Scaffold Seeded with a Co-Culture of Schwann Cells and Adipose-Derived Stem Cells for Sciatic Nerve Regeneration. PLoS One. 2016;11(1):e147184. [32]Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30(15): 2956-2965. [33]Mikelsaar AV, Sunter A, Mikelsaar R, et al. Epitope of titin A-band-specific monoclonal antibody Tit1 5 H1.1 is highly conserved in several Fn3 domains of the titin molecule. Centriole staining in human, mouse and zebrafish cells. Cell Div. 2012;7(1):21. [34]Lim JS, Ki CS, Kim JW, et al. Fabrication and evaluation of poly(epsilon-caprolactone)/silk fibroin blend nanofibrous scaffold. Biopolymers. 2012;97(5): 265-275. [35]Kemp SW, Syed S, Walsh W, et al. Collagen nerve conduits promote enhanced axonal regeneration, schwann cell association, and neovascularization compared to silicone conduits. Tissue Eng Part A. 2009;15(8):1975-1988. [36]Schnell E, Klinkhammer K, Balzer S, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012-3025. [37]Plant AL, Bhadriraju K, Spurlin TA, et al. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta. 2009;1793(5):893-902. [38]De Nicola AF, Labombarda F, Gonzalez DM, et al. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol. 2009;30(2):173-187. [39]Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718-726. [40]Bhardwaj N, Chakraborty S, Kundu SC. Freeze-gelled silk fibroin protein scaffolds for potential applications in soft tissue engineering. Int J Biol Macromol. 2011;49(3): 260-267. [41]Uebersax L, Merkle HP, Meinel L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Control Release. 2008;127(1):12-21. [42]Bhardwaj N, Chakraborty S, Kundu SC. Freeze-gelled silk fibroin protein scaffolds for potential applications in soft tissue engineering. Int J Biol Macromol. 2011; 49(3):260-267. [43]Yeong WY, Chua CK, Leong KF, et al. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004;22(12):643-652. [44]Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24(13):2363-2378. [45]Curtis AS. Small is beautiful but smaller is the aim: review of a life of research. Eur Cell Mater. 2004;8: 27-36. [46]Park KD, Wang X, Lee JY, et al. Research trends in biomimetic medical materials for tissue engineering: commentary. Biomater Res. 2016;20:8. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||