Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (36): 5440-5449.doi: 10.3969/j.issn.2095-4344.2016.36.018
Previous Articles Next Articles
Induced pluripotent stem cells and cardiovascular disease
Zhan Qi, Zhang Jing, Wang Xiao-qing, Chen Xiao-fang, Huang Yan
- Key Laboratory for Biomechanics and Mechanobiology of Ministry of Education, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, China
-
Revised:2016-07-29Online:2016-09-02Published:2016-09-02 -
Contact:Huang Yan, Ph.D., Lecturer, Master’s supervisor, Key Laboratory for Biomechanics and Mechanobiology of Ministry of Education, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, China -
About author:Zhan Qi, Studying for master’s degree, Key Laboratory for Biomechanics and Mechanobiology of Ministry of Education, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, China -
Supported by:the National Natural Science Foundation of China, No. 11302020
CLC Number:
Cite this article
Zhan Qi, Zhang Jing, Wang Xiao-qing, Chen Xiao-fang, Huang Yan. Induced pluripotent stem cells and cardiovascular disease[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(36): 5440-5449.
share this article
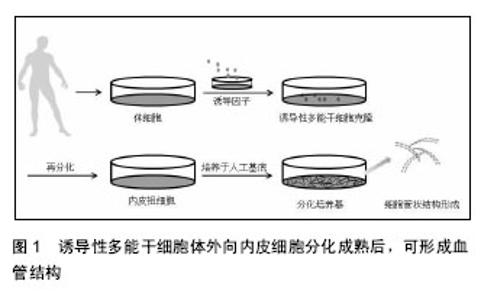
2.1 诱导性多能干细胞向心肌细胞方向的分化 2.1.1 诱导性多能干细胞向心肌细胞方向分化的方法 诱导性多能干细胞向心肌细胞方向分化时,通常先诱导形成拟胚体,拟胚体是胚胎干细胞在悬浮培养条件下形成的具有三维结构的细胞团,细胞构成与发育中的胚胎相似,包括内、中、外3种初始胚层,再转到分化培养基进行定向分化[2-3]。Honda等[4]将悬浮培养的拟胚体植入凝胶层上,在DMEM/F12中加入体积分数为20%的胎牛血清、L-谷氨酰胺、非必须氨基酸、β-巯基乙醇、青霉素和链霉素,1周后细胞出现了收缩现象。Kawamura等[5]、Tanaka等[6]和Dambrot等[7]也用添加了一些其他因子的DMEM培养基,成功分化出心肌细胞。Mauritz等[8]则以IMDM为基础,将拟胚体分化为心肌细胞。Burridge等[9]开发了一种通用的诱导性多能干细胞高效稳定分化为心肌细胞的系统,在拟胚体形成后,将细胞分散培养在V型底的96孔板中,用以1640为基础的分化培养基诱导其分化为心肌细胞。Zwi-Dantsis等[10]用搅拌式培养系统使诱导性多能干细胞直接形成拟胚体进而分化,细胞依次表达了中胚层标志物、心肌转录因子、心肌细胞结构基因,此法与悬滴法形成的拟胚体基因表达谱系相似,但简化了步骤。 有效的诱导性多能干细胞向心肌方向分化往往需要在培养基中加入一些促进因子,包括环孢菌素A、维生素C、血管内皮生长因子、骨形态发生蛋白4、成纤维细胞生长因子和Wnt通路抑制因子(Dickkopf-1,Dkk1)等。Fujiwara等[11]研究发现环孢菌素A不仅促进小鼠诱导性多能干细胞向心肌细胞的分化,也能促进心脏祖细胞的形成,在血管内皮细胞生长因子受体2(kinase insert domain receptor,KDR)阳性细胞分化的过程中加入环孢菌素A可以使心肌肌钙蛋白T阳性心肌细胞上升12倍,增强心肌的自发收缩和动作电位,并且在这些肌钙蛋白T阳性细胞中能检测到钙通道和心室离子通道。Cao等[12]证实了维生素C通过促进心脏祖细胞的增殖,可提高诱导性多能干细胞向心肌细胞的分化效率。Ye等[13]也证实了血管内皮生长因子能够促进诱导性多能干细胞的心肌向分化。在凝胶层的培养基中,补充谷氨酸盐、硫代甘油、抗坏血酸、维生素C、骨形态发生蛋白4,并在分化的不同时段加入适当的细胞活素类物质如Dkk1、血管内皮生长因子、成纤维细胞生长因子,最终可以得到心肌细胞[14-17]。Uosaki等[18] 改进了培养方式,使诱导性多能干细胞分散后培养在鼠胚胎成纤维细胞饲养层中并加入成纤维细胞生长因子,诱导分化时换成1640培养基,分时段补充激活素 A、Dkk1等相应因子培养后可观察到心肌基因表达。除了以上的因子,Correia等[19]发现诱导性多能干细胞在含氧4%的低氧环境比含氧20%的环境中心肌细胞产量高出1 000倍,并且分化速度也较快,在分化第11天即可检测到心肌细胞的特征。 总之,诱导性多能干细胞可以向心肌细胞方向分化,诱导性多能干细胞的质量与心肌向分化的效率有着密切的关系,选择高质量诱导性多能干细胞会带来更好的分化效果[20]。在诱导性多能干细胞心肌向分化的过程中,适当的基础培养基、适量的细胞活性因子都是决定分化效果的重要因素,各种创新优化的方法也使得分化效率越来越高[21]。 2.1.2 诱导性多能干细胞源心肌细胞的生理特点 未分化的诱导性多能干细胞具有一些干性基因,分化为心肌细胞后有些干性基因会消失,相应的心肌细胞特异性表达物会出现,例如α-肾上腺素能1A受体及其mRNA在未分化的人诱导性多能干细胞中大量存在,但是分化为心肌细胞后消失[22]。诱导性多能干细胞源心肌细胞主要的特异性标志物包括α-辅肌动蛋白、肌动蛋白、肌钙蛋白T、肌球蛋白重链6、肌球蛋白轻链2、Nkx2-5、Gata4等[5,17]。搏动的拟胚体细胞也可表达肌钙蛋白I、肌钙蛋白T、α-辅肌动蛋白、整合素43[10]。 心肌细胞的正常生理收缩功能与电生理特性密切相关,涉及到以钙离子为主的离子通道,钙离子的活动表现出搏动的自律性[8]。虽然诱导性多能干细胞向心肌细胞方向分化后的细胞表达心肌相关的一些基因,但其成熟性有限并且分化程度多样,有些并不具有完全的成熟生理功能,如对钙火花的检测发现细胞器局部重复的钙火花,意味着细胞分化不够成熟[23]。因此促进细胞的进一步发育生长也是一个研究重点,研究表明,神经调节蛋白1β和三碘甲腺原氨酸可促进心肌细胞发育成熟[24-25]。诱导性多能干细胞源心肌细胞能够建立起激素调节通路和功能性离子通道并具有心室表型,可能在不完全重编程的情况下,早期搏动频率很低、成熟时间晚于胚胎干细胞源心肌细胞[26],其自发收缩机制是由于钙离子活动引起的,钙离子在肌质网的活动与动作电位相关[27]。Lee等[14]得到的诱导性多能干细胞源心肌细胞的α-肌球蛋白重链和Nkx2-5的表达量可与胚胎干细胞源心肌细胞相媲美,但其钙处理能力相对不成熟,并且活动幅度较小。Germanguz等[28]分析了诱导性多能干细胞源心肌细胞的分子表征和功能特性,表明诱导性多能干细胞源心肌细胞可以表达心脏特异性的基因和蛋白,与成体心肌细胞相比,其搏动频率和力度较弱,对兰尼碱和咖啡因处理的反应不是很强烈,但仍表达肌质网钙离子处理相关蛋白,总体来说其生理特性类似于成体心肌细胞。 诱导性多能干细胞源心肌细胞的肌丝沿着长轴生长,但是阿霉素处理后,肌原纤维序列混乱,这与胚胎干细胞源心肌细胞相同[15],但诱导性多能干细胞源心肌细胞具有更高的不稳定性。Burridge等[9]的分化系统可以提高非病毒、非整合游离基因的成纤维细胞源诱导性多能干细胞心肌向的稳定分化。Földes等[22]得到的诱导性多能干细胞源心肌细胞没有表达肥厚所需的感受器苯肾上腺素和内皮素1。此外,在诱导性多能干细胞中可以检测到包括α-半乳糖在内的几种多糖的存在,在分化为心肌细胞后N-多糖的结构发生了变化[29]。 总之,诱导性多能干细胞源心肌细胞虽然表达心肌细胞的特异性基因,大部分的生理特性与胚胎干细胞源心肌细胞相同,但是仍然具有一些不足之处,如重复钙火花、搏动频率低、基因表达缺陷等都表明诱导性多能干细胞源心肌细胞的分化并不完全成熟。经检测,一些诱导性多能干细胞源心肌细胞与心肌细胞或者胚胎干细胞源心肌细胞相比,表型有所不同,这可能与诱导性多能干细胞本身的潜能有关[30],也可能与分化方法有关,但其基本功能与特征没有本质上的不同,分化进程与胚胎干细胞基本一致[31]。 2.2 诱导性多能干细胞向内皮细胞方向的分化 2.2.1 诱导性多能干细胞向内皮细胞方向分化的方法 诱导性多能干细胞分化为内皮细胞的方式有多种,有3D培养拟胚体然后再进行内皮向分化,也有与OP9细胞共培养等方式[32]。血管内皮生长因子可以诱导悬浮培养的拟胚体为内皮细胞[33]。也可将拟胚体在人工基底膜形成CD34阳性的内皮祖细胞,再接种在明胶涂层上,用内皮细胞生长培养基2和基础培养基2混合培养,仍可诱导为内皮细胞[34]。用CD34、神经黏蛋白、KDR筛选的内皮祖细胞也可进一步培养成内皮细胞[35]。以血管内皮生长因子为主要诱导剂时,适时适量的补充骨形态发生蛋白4、成纤维细胞生长因子等是内皮向分化的常见方法[36-38],细胞可直接分化,也可通过中胚层、内皮祖细胞的过渡间接分化[39-40]。如骨形态发生蛋白4和血管内皮生长因子在无血清培养基中可以诱导生成CD31阳性VE-Cadherin阳性内皮细胞[32]。另外,血管生成素1能够促进CD41阳性细胞和KDR阳性中胚层前体细胞内皮向分化,它是通过提高Flk-1阳性中胚层前体细胞中Tie2-PI3K-Akt 的活性来促进内皮向分化[41]。Notch信号抑制剂可能促进诱导性多能干细胞的内皮向分化,在抑制Notch通路时,内皮标志物CD31和血管内皮钙黏蛋白(vascular endothelium cadherin,VE-Cadherin)显著上升[42],MicroRNA-21和转化生长因子β2的信号通路可能调控诱导性多能干细胞分化为内皮细胞的过程[43]。 2.2.2 诱导性多能干细胞源内皮细胞的生理特点 通过免疫组化、Western bloting、RT-PCR等方法可观察到诱导性多能干细胞源内皮细胞的一些生理特征。由诱导性多能干细胞形成的CD34阳性细胞会大量表达中胚层标记(Brachyury (T)、MSX1、IGF2和Runx2)和内皮祖细胞标记(CD34、CD31、KDR和VE-Cadherin),进一步分化为成熟内皮细胞后,形态发生改变,各标志物如血管性血友病因子、VE-Cadherin、CD31、CD105先后出现并增多[34]。血管内皮生长因子作为促进因子,具有十分明显的作用,可以使VE-Cadherin和CD31的表达显著上升[40]。Adams等[44]发现0-18 d,内皮细胞标志物CD31、KDR和VE-Cadherin的表达都不断上升,在第10天VE-Cadherin阳性/CD31阳性细胞达到峰值。与胚胎干细胞源内皮细胞相比,诱导性多能干细胞源心肌细胞表达出更多的CD54,但是CD106和Flk-1的水平更低些。内皮型一氧化氮合酶也是内皮细胞的一个主要特征,可用于鉴定分化结果[33]。 2.2.3 诱导性多能干细胞源内皮细胞体外形成血管结构 诱导性多能干细胞体外内皮向分化成熟后,可形成血管结构(图1)。Margariti等[45]用血管内皮生长因子等因子处理,可以提高VE-Cadherin的表达水平,所形成的内皮细胞具有很好的黏附性和稳定性,在支架上培养可形成典型的管状结构。CD34阳性内皮祖细胞在VEGF-A存在的基质胶上培养能够形成管状结构[46];KDR阳性细胞培养在胶原涂层上可生长为成熟的血管细胞,体外三维培养也可以形成血管的网架结构[47]。Choi等[36]将诱导性多能干细胞与OP9共培养,经流式细胞术得到了CD31阳性CD43阴性细胞,并将其培养在纤黏连蛋白涂层培养板上,加入成纤维细胞生长因子、血管内皮生长因子等因子,以VE-Cadherin抗体染色做指示剂进行了血管生成实验。Belair等[48]利用诱导性多能干细胞源内皮细胞进行一系列实验证实其在体外适宜环境中可形成可靠的血管结构模型。除验证了内皮细胞的基本功能,还通过加载剪切力和微流动腔实验使其整齐的排列,在人工基底膜上的2D、3D培养均可形成毛细血管网结构。 总之,诱导性多能干细胞源内皮细胞除具有特异性基因和表达物外,在分化成熟后可在适当条件下形成血管样结构,这也是观察内皮细胞特性的重要指标。血管内皮生长因子信号通路在干细胞内皮向分化的过程中并非必要,但是在内皮细胞的存活方面可能具有重要作用。骨形成蛋白执行Hedgehog信号通路的下游功能,对血管发生具有重要的调控作用,骨形态发生蛋白4可以促进胚胎中胚层发育和内皮祖细胞的形成,通过SMAD1/5信号通路诱导胚胎干细胞转化为KDR阳性细胞[49]。"
| [1] Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [2] Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88-95. [3] 李晴,刘靖,徐秀琴.人类多能干细胞源心肌细胞的研究进展与应用前景[J].中国细胞生物学学报,2013,35(3): 251-261. [4] Honda M, Kiyokawa J, Tabo M, et al. Electrophysiological characterization of cardiomyocytes derived from human induced pluripotent stem cells. J Pharmacol Sci. 2011;117(3):149-159. [5] Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(11 Suppl 1):S29-37. [6] Tanaka A, Yuasa S, Mearini G, et al. Endothelin-1 induces myofibrillar disarray and contractile vector variability in hypertrophic cardiomyopathy-induced pluripotent stem cell-derived cardiomyocytes. J Am Heart Assoc. 2014;3(6):e001263. [7] Dambrot C, Braam SR, Tertoolen LG, et al. Serum supplemented culture medium masks hypertrophic phenotypes in human pluripotent stem cell derived cardiomyocytes. J Cell Mol Med. 2014;18(8):1509-1518. [8] Mauritz C, Schwanke K, Reppel M, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118(5): 507-517. [9] Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011 8;6(4):e18293. [10] Zwi-Dantsis L, Mizrahi I, Arbel G, et al. Scalable production of cardiomyocytes derived from c-Myc free induced pluripotent stem cells. Tissue Eng Part A. 2011;17(7-8):1027-1037. [11] Fujiwara M, Yan P, Otsuji TG, et al. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS One. 2011;6(2):e16734. [12] Cao N, Liu Z, Chen Z, et al. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012;22(1):219-236. [13] Ye L, Zhang S, Greder L, et al. Effective cardiac myocyte differentiation of human induced pluripotent stem cells requires VEGF. PLoS One. 2013;8(1): e53764. [14] Lee YK, Ng KM, Lai WH, et al. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes.Stem Cell Rev. 2011;7(4):976-986. [15] Shinozawa T, Furukawa H, Sato E, et al. A novel purification method of murine embryonic stem cell- and human-induced pluripotent stem cell-derived cardiomyocytes by simple manual dissociation. J Biomol Screen. 2012;17(5):683-691. [16] Lan F, Lee AS, Liang P, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12(1): 101-113. [17] Shinozawa T, Imahashi K, Sawada H, et al. Determination of appropriate stage of human-induced pluripotent stem cell-derived cardiomyocytes for drug screening and pharmacological evaluation in vitro. J Biomol Screen. 2012;17(9):1192-1203. [18] Uosaki H, Fukushima H, Takeuchi A, et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011;6(8): e23657. [19] Correia C, Serra M, Espinha N, et al. Combining hypoxia and bioreactor hydrodynamics boosts induced pluripotent stem cell differentiation towards cardiomyocytes. Stem Cell Rev. 2014;10(6):786-801. [20] Ohno Y, Yuasa S, Egashira T, et al. Distinct iPS Cells Show Different Cardiac Differentiation Efficiency. Stem Cells Int. 2013;2013:659739. [21] Fujita J, Fukuda K. Future prospects for regenerated heart using induced pluripotent stem cells. J Pharmacol Sci. 2014;125(1):1-5. [22] Földes G, Matsa E, Kriston-Vizi J, et al. Aberrant α-adrenergic hypertrophic response in cardiomyocytes from human induced pluripotent cells. Stem Cell Reports. 2014;3(5):905-914. [23] Zhang GQ, Wei H, Lu J, et al. Identification and characterization of calcium sparks in cardiomyocytes derived from human induced pluripotent stem cells. PLoS One. 2013;8(2):e55266. [24] Iglesias-García O, Baumgartner S, Macrí-Pellizzeri L, et al. Neuregulin-1β induces mature ventricular cardiac differentiation from induced pluripotent stem cells contributing to cardiac tissue repair. Stem Cells Dev. 2015;24(4):484-496. [25] Yang X, Rodriguez M, Pabon L, et al. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296-304. [26] Kuzmenkin A, Liang H, Xu G, et al. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23(12):4168-4180. [27] Kim JJ, Yang L, Lin B, et al. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J Mol Cell Cardiol. 2015;81: 81-93. [28] Germanguz I, Sedan O, Zeevi-Levin N, et al. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2011;15(1):38-51. [29] Kawamura T, Miyagawa S, Fukushima S, et al. N-glycans: phenotypic homology and structural differences between myocardial cells and induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014;9(10):e111064. [30] Kaichi S, Hasegawa K, Takaya T, et al. Cell line-dependent differentiation of induced pluripotent stem cells into cardiomyocytes in mice. Cardiovasc Res. 2010;88(2):314-323. [31] Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225-229. [32] Tan KS, Tamura K, Lai MI, et al. Molecular pathways governing development of vascular endothelial cells from ES/iPS cells. Stem Cell Rev. 2013;9(5):586-598. [33] Sivarapatna A, Ghaedi M, Le AV, et al. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials. 2015;53:621-633. [34] Yoo CH, Na HJ, Lee DS, et al. Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials. 2013;34(33):8149-8160. [35] Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110(31):12774-12779. [36] Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3): 559-567. [37] Homma K, Sone M, Taura D, et al. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells. Atherosclerosis. 2010;212(1):42-47. [38] White MP, Rufaihah AJ, Liu L, et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31(1):92-103. [39] Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118(5):498-506. [40] Du C, Narayanan K, Leong MF, et al. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 2014;35(23): 6006-6014. [41] Joo HJ, Kim H, Park SW, et al. Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells. Blood. 2011;118(8):2094-2104. [42] Lee JB, Werbowetski-Ogilvie TE, Lee JH, et al. Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent stem cells. Blood. 2013;122(7):1162-1173. [43] Di Bernardini E, Campagnolo P, Margariti A, et al. Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor β2 (TGF-β2) pathways. J Biol Chem. 2014;289(6):3383-3393. [44] Adams WJ, Zhang Y, Cloutier J, et al. Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports. 2013; 1(2):105-113. [45] Margariti A, Winkler B, Karamariti E, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109(34):13793-13798. [46] Xu Y, Liu L, Zhang L, et al. Efficient commitment to functional CD34+ progenitor cells from human bone marrow mesenchymal stem-cell-derived induced pluripotent stem cells. PLoS One. 2012;7(4): e34321. [47] Suzuki H, Shibata R, Kito T, et al. Comparative angiogenic activities of induced pluripotent stem cells derived from young and old mice. PLoS One. 2012; 7(6): e39562. [48] Belair DG, Whisler JA, Valdez J, et al. Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev. 2015;11(3):511-525. [49] Zhou Y, Yang F, Chen T, et al. An updated view on the differentiation of stem cells into endothelial cells. Sci China Life Sci. 2014;57(8):763-773. [50] Gu M, Nguyen PK, Lee AS, et al. Microfluidic single-cell analysis shows that porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation. Circ Res. 2012;111(7):882-893. [51] Mauritz C, Martens A, Rojas SV, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J. 2011;32(21):2634-2641. [52] Suzuki H, Shibata R, Kito T, et al. Therapeutic angiogenesis by transplantation of induced pluripotent stem cell-derived Flk-1 positive cells. BMC Cell Biol. 2010;11:72. [53] Prasain N, Lee MR, Vemula S, et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol. 2014;32(11):1151-1157. [54] Morita R, Suzuki M, Kasahara H, et al. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A. 2015;112(1):160-165. [55] Kim KL, Song SH, Choi KS, et al. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng Part A. 2013;19(21-22):2478-2485. [56] Templin C, Zweigerdt R, Schwanke K, et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126(4):430-439. [57] Citro L, Naidu S, Hassan F, et al. Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction. PLoS One. 2014; 9(12):e116281. [58] Masumoto H, Ikuno T, Takeda M, et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4:6716. [59] Wang Y, Huang W, Liang J, et al. Suicide gene-mediated sequencing ablation revealed the potential therapeutic mechanism of induced pluripotent stem cell-derived cardiovascular cell patch post-myocardial infarction. Antioxid Redox Signal. 2014;21(16):2177-2191. [60] Carpenter L, Carr C, Yang CT, et al. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21(6):977-986. [61] Miki K, Uenaka H, Saito A, et al. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl Med. 2012;1(5):430-437. [62] Matsa E, Dixon JE, Medway C, et al. Allele-specific RNA interference rescues the long-QT syndrome phenotype in human-induced pluripotency stem cell cardiomyocytes. Eur Heart J. 2014;35(16):1078-1087. [63] Mehta A, Sequiera GL, Ramachandra CJ, et al. Re-trafficking of hERG reverses long QT syndrome 2 phenotype in human iPS-derived cardiomyocytes. Cardiovasc Res. 2014;102(3):497-506. [64] Fatima A, Kaifeng S, Dittmann S, et al. The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PLoS One. 2013;8(12): e83005. [65] Hick A, Wattenhofer-Donzé M, Chintawar S, et al. Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich's ataxia. Dis Model Mech. 2013 May;6(3):608-621. [66] Caspi O, Huber I, Gepstein A, et al. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ Cardiovasc Genet. 2013;6(6):557-568. [67] Drawnel FM, Boccardo S, Prummer M, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810-821. [68] Lin B, Li Y, Han L, et al. Modeling and study of the mechanism of dilated cardiomyopathy using induced pluripotent stem cells derived from individuals with Duchenne muscular dystrophy. Dis Model Mech. 2015; 8(5):457-466. [69] Sharma A, Marceau C, Hamaguchi I, et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ Res. 2014;115(6):556-566. [70] Doherty KR, Talbert DR, Trusk PB, et al. Structural and functional screening in human induced-pluripotent stem cell-derived cardiomyocytes accurately identifies cardiotoxicity of multiple drug types. Toxicol Appl Pharmacol. 2015;285(1):51-60. [71] Hayakawa T, Kunihiro T, Ando T, et al. Image-based evaluation of contraction-relaxation kinetics of human-induced pluripotent stem cell-derived cardiomyocytes: Correlation and complementarity with extracellular electrophysiology. J Mol Cell Cardiol. 2014;77:178-191. [72] Nakamura Y, Matsuo J, Miyamoto N, et al. Assessment of testing methods for drug-induced repolarization delay and arrhythmias in an iPS cell-derived cardiomyocyte sheet: multi-site validation study. J Pharmacol Sci. 2014;124(4):494-501. [73] Lu J, Wei H, Wu J, et al. Evaluation of the cardiotoxicity of mitragynine and its analogues using human induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014;9(12):e115648. [74] Han L, Li Y, Tchao J, et al. Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells.Cardiovasc Res. 2014;104(2): 258-269. [75] Nozaki Y, Honda Y, Tsujimoto S, et al. Availability of human induced pluripotent stem cell-derived cardiomyocytes in assessment of drug potential for QT prolongation. Toxicol Appl Pharmacol. 2014;278(1): 72-77. [76] Rao C, Prodromakis T, Kolker L, et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34(10): 2399-2411. [77] Rodriguez ML, Graham BT, Pabon LM, et al. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J Biomech Eng. 2014;136(5):051005. [78] Josowitz R, Lu J, Falce C, et al. Identification and purification of human induced pluripotent stem cell-derived atrial-like cardiomyocytes based on sarcolipin expression. PLoS One. 2014;9(7):e101316. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [4] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [5] | Jiang Xin, Qiao Liangwei, Sun Dong, Li Ming, Fang Jun, Qu Qingshan. Expression of long chain non-coding RNA PGM5-AS1 in serum of renal transplant patients and its regulation of human glomerular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 741-745. |
| [6] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [7] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [8] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [9] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [10] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [11] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [12] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [13] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [14] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [15] | Zhou Wu, Wang Binping, Wang Yawen, Cheng Yanan, Huang Xieshan. Transforming growth factor beta combined with bone morphogenetic protein-2 induces the proliferation and differentiation of mouse MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3630-3635. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||