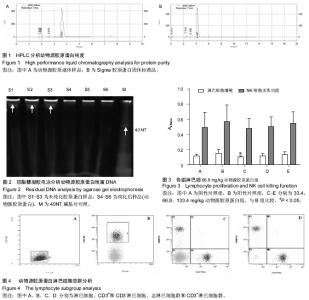
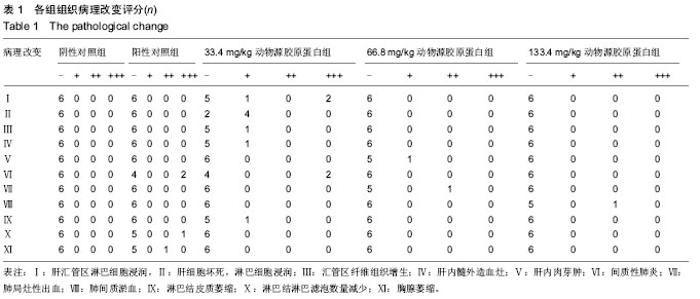
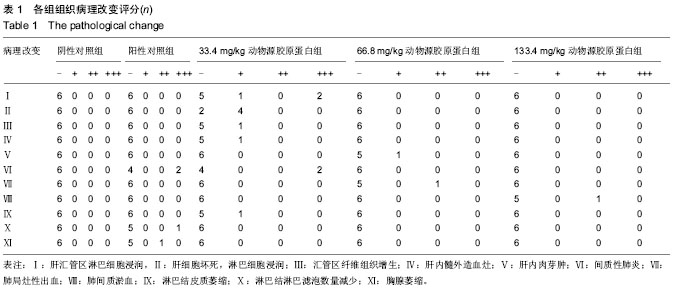
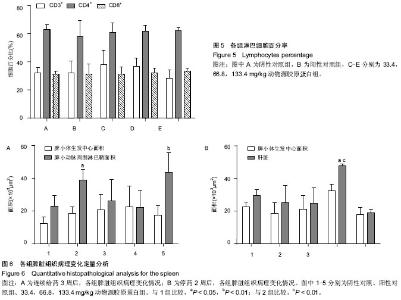
[1] Aragona F.Is bovine collagen safe?J Urol (Paris).1991;97(6): 279-281.
[2] Cooperman L,Michaeli D.The immunogenicity of injectable collagen. II. A retrospective review of seventy-two tested and treated patients. J Am Acad Dermatol. 1984;10(4):647-651.
[3] Mcclelland M,Egbert B,Hanko V,et al.Evaluation of artecoll polymethylmethacrylate implant for soft-tissue augmentation: biocompatibility and chemical characterization.Plast Reconstr Surg.1997;100(6):1466-1474.
[4] Beil W,Timpl R,Furthmayr H.Conformation dependence of antigenic determinants on the collagen molecule.Immunology. 1973;24(1):13-24.
[5] Dahm M,Lyman WD,Schwell AB,et al.Immunogenicity of glutaraldehyde-tanned bovine pericardium.J Thorac Cardiovasc Surg.1990;99(6):1082-1090.
[6] Courtman DW,Errett BF,Wilson GJ.The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices.J Biomed Mater Res. 2001;55(4):576-586.
[7] Bondioli E, Fini M,Veronesi F,et al.Development and evaluation of a decellularized membrane from human dermis. J Tissue Eng Regen Med.2014;8(4):325-336.
[8] Moore MA,Samsell B,Wallis G,et al.Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review.Cell Tissue Bank.2015;16(2): 249-259.
[9] Torudd J,Protopopova M,Sarimov R,et al.Dose-response for radiation-induced apoptosis, residual 53BP1 foci and DNA-loop relaxation in human lymphocytes.Int J Radiat Biol. 2005;81(2):125-138.
[10] Al Arfaj AS,Chowdhary AR,Khalil N,et al.Immunogenicity of singlet oxygen modified human DNA: implications for anti-DNA antibodies in systemic lupus erythematosus. Clin Immunol.2007;124(1):83-89.
[11] Umthong S,Buaklin A,Jacquet A,et al.Immunogenicity of a DNA and Recombinant Protein Vaccine Combining LipL32 and Loa22 for Leptospirosis Using Chitosan as a Delivery System.J Microbiol Biotechnol.2015;25(4):526-536.
[12] Mackenzie-Dyck S,Latimer L,Atanley E,et al.Immunogenicity of a bovine herpesvirus 1 glycoprotein D DNA vaccine complexed with bovine neutrophil beta-defensin 3. Clin Vaccine Immunol.2015;22(1):79-90.
[13] Furuzawa-Carballeda J,Rojas E,Valverde M,et al. Cellular and humoral responses to collagen-polyvinylpyrrolidone administered during short and long periods in humans. Can J Physiol Pharmacol.2003;81(11):1029-1035.
[14] Vierboom MP,Breedveld E,Kondova I,et al.Collagen-induced arthritis in common marmosets: a new nonhuman primate model for chronic arthritis.Arthritis Res Ther. 2010; 12(5): R200.
[15] Garcia-Domingo MI,Alijotas-Reig J,Cistero-Bahima A,et al.Disseminated and recurrent sarcoid-like granulomatous panniculitis due to bovine collagen injection. J Investig Allergol Clin Immunol.2000;10(2):107-109.
[16] Solinger AM, Bhatnagar R, Stobo JD.Cellular, molecular, and genetic characteristics of T cell reactivity to collagen in man.Proc Natl Acad Sci U S A.1981;78(6):3877-3881.