Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (20): 3130-3137.doi: 10.3969/j.issn.2095-4344.3135
Previous Articles Next Articles
Bmal1 and Clock regulate the development and differentiation of skeletal muscle
Yang Xinhua1, Yan Yindi1, Luo Xuguang2, Yang Yanping1, Li Hairong1, Cui Huilin1, Cao Ximei1
- 1Department of Histology and Embryology, 2Department of Microbiology and Immunology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2019-12-23Revised:2019-12-28Accepted:2020-07-26Online:2021-07-18Published:2021-01-15 -
Contact:Cao Ximei, Associate professor, Master’s supervisor, Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Yang Xinhua, Master candidate, Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:the Natural Science Foundation of Shanxi Province, No. 201901D111185 (to CXM); the Natural Science Foundation of Shanxi Province for the Youth, No. 2014021028-1 (to CXM); the Science and Technology Innovation Foundation of Shanxi Medical University, No. 01201401 (to CXM
CLC Number:
Cite this article
Yang Xinhua, Yan Yindi, Luo Xuguang, Yang Yanping, Li Hairong, Cui Huilin, Cao Ximei. Bmal1 and Clock regulate the development and differentiation of skeletal muscle[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3130-3137.
share this article
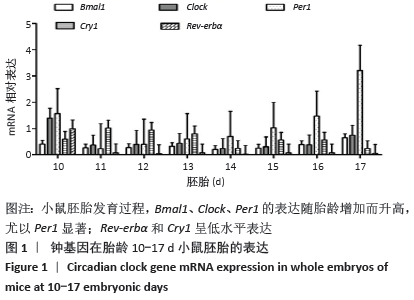
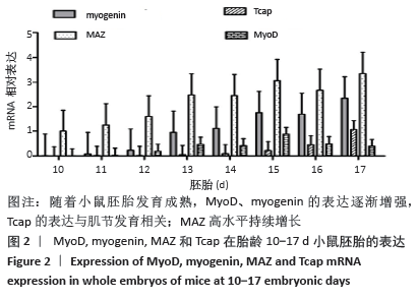
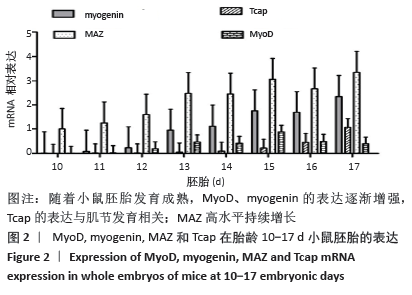
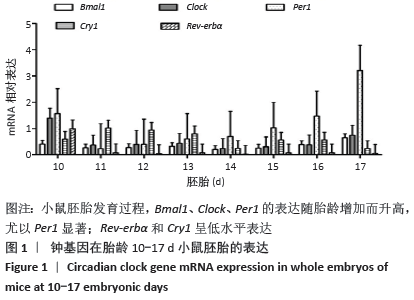
2.1 核心钟基因在不同胎龄小鼠胚胎的表达 小鼠胚胎发育早期胎龄10 d,核心钟基因Bmal1、Clock、Cry1、Per1和Rev-erbα相对高表达,可能是受孕鼠子宫组织的影响(图1)。随胎龄增加,Rev-erbα的表达水平仅略高于基线。小鼠胚胎发育11-12 d,钟基因 Bmal1、Clock和Per1的相对表达水平均比较低,胎龄15 d始,三者的表达开始升高,尤以Per1显著;胎龄17 d,Per1的表达陡然升高,约达胎龄15 d表达水平的3倍以上。Bmal1和Clock维持低水平表达直至胎龄15 d,小鼠胚胎发育后期16-17 d,二者的表达水平仅有小幅升高。胎龄11-13 d,Cry1的表达水平相对高于Bmal1、Clock、Per1和Rev-erbα,随后小幅下降;至胎龄17 d,Cry1的表达水平仅略高于Rev-erbα。 "
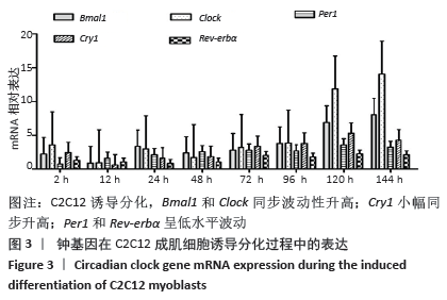
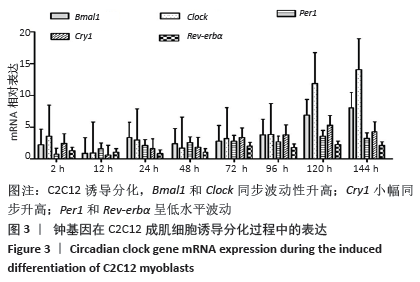
骨骼肌的基本结构和功能单位肌节由Z线(Z-disc)分隔,Tcap(Tintin cap)参与构成Z线。小鼠胚胎发育早期Tcap的表达接近基线,胎龄13 d始有略高于基线的微弱表达;胎龄15 d,Tcap的表达明显增高,并随着胎龄增加同步升高,升高趋势和myogenin相一致。小鼠胚胎发育过程中,MAZ始终维持高水平表达,且随着胎龄增加进一步波动性升高。 2.3 核心钟基因在C2C12成肌细胞诱导分化过程中的表达 核心钟基因Bmal1、Clock、Per1、Cry1和Rev-erbα在C2C12成肌细胞诱导分化前仅高于基线的弱表达(图未示)。诱导分化2 h,上述核心钟基因均有低水平的表达,Clock较其他核心钟基因表达水平高。随着诱导分化时间延长,Bmal1和Clock同步呈波动性升高。诱导分化96 h,Bmal1和Clock陡然增高,且Clock显著超过Bmal1快速增加;诱导分化 144 h,Clock的表达水平达Bmal1的1.5倍以上。随Bmal1和Clock表达波动性升高,Cry1小幅同步升高;诱导分化120 h达小高峰而后下降,诱导分化144 h表达水平和96 h将近一致。C2C12细胞诱导分化过程中,Per1表达水平仅略高于Rev-erbα,二者呈小幅低水平波动(图3)。 "
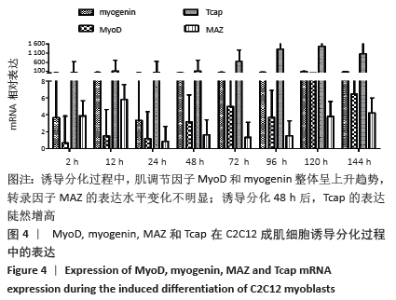
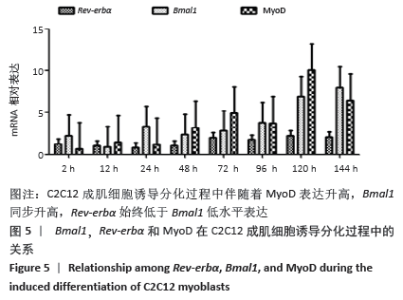
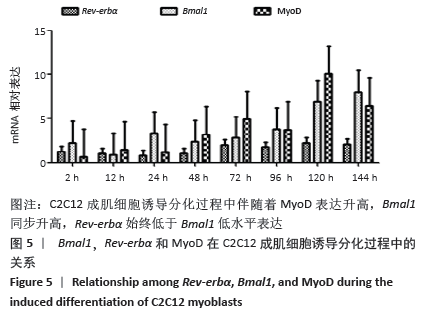
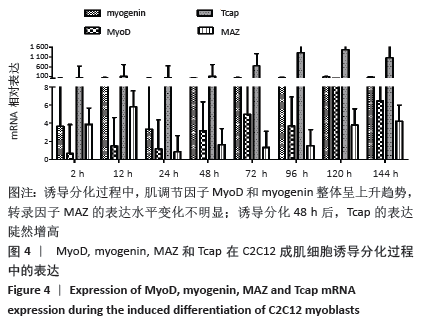
2.4 肌调节因子MyoD和myogenin在C2C12成肌细胞诱导分化过程中的表达 C2C12成肌细胞诱导分化前肌调节因子MyoD和myogenin未表达;诱导分化过程中,肌调节因子MyoD和myogenin整体呈上升趋势,随着诱导分化时间延长myogenin升高趋势更显著;诱导分化48 h后,MyoD出现小幅升高。此时,Tcap的表达陡然增高,并随诱导时间延长同步升高,提示肌节快速发育。在C2C12成肌细胞诱导分化过程中,转录因子MAZ的表达水平变化不明显(图4)。C2C12成肌细胞诱导分化过程中伴随着MyoD表达升高,Bmal1同步升高,48 h后,MyoD出现小幅升高Bmal1表达时快速增加,Rev-erbα始终低于Bmal1低水平表达(图5)。 "
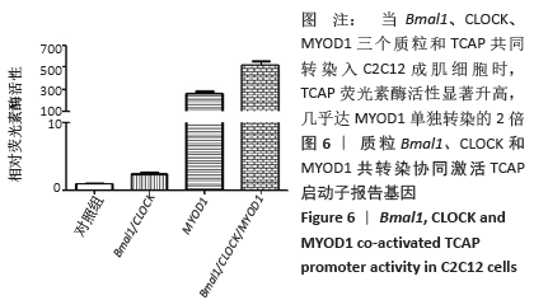
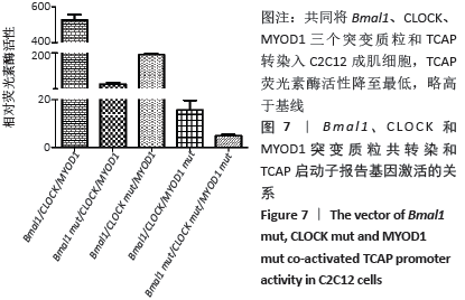
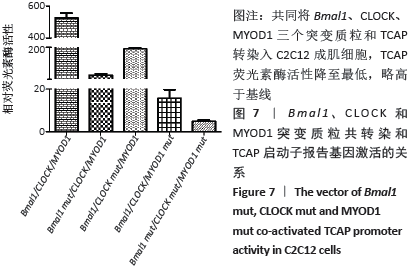
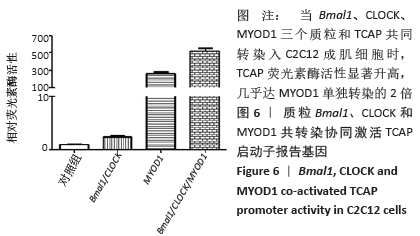
荧光素酶活性分析实验表明,C2C12成肌细胞转染MyoD1和TCAP,TCAP荧光素酶活性明显增加,MyoD1显著激活TCAP;质粒Bmal1、Clock、TCAP共转染,对TCAP有弱的激活作用;当Bmal1、Clock、MyoD1三个质粒和TCAP共同转染入C2C12成肌细胞时,TCAP荧光素酶活性显著升高,几乎达MyoD1单独转染的2倍(图6)。按实验设计分组,分别将Bmal1、Clock、MyoD1突变质粒和TCAP共同转染入C2C12成肌细胞,TCAP荧光素酶活性均显著下调(图7);共同将Bmal1、Clock、MyoD1三个突变质粒和TCAP转染入C2C12成肌细胞,TCAP荧光素酶活性降至最低,略高于基线(图7)。 "
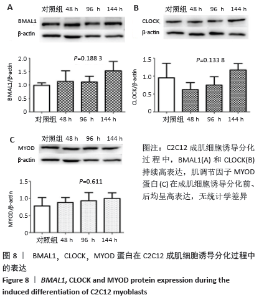
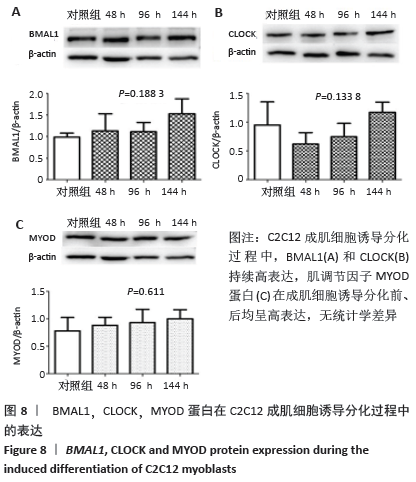
2.5 C2C12成肌细胞诱导分化过程中Bmal1、Clock和MyoD1蛋白的表达 C2C12成肌细胞诱导分化前,Bmal1和Clock都有高表达(图8)。C2C12成肌细胞诱导分化过程中,Bmal1和Clock持续高表达,Bmal1表达条带较Clock更深染,但是二者持续高表达没有表现出时间依赖性(P=0.188 3> 0.05,P=0.133 8> 0.05,图8A,B)。肌调节因子MyoD1蛋白水平的表达在成肌细胞诱导分化前、后均呈高表达;随诱导分化时间延长,高表达的MyoD1蛋白较诱导分化前没有统计学差异(P=0.611> 0.05,图8C),这与其他学者的研究不一致[9-10]。肌分化过程复杂,可能是受某些因素影响的结果。已知MyoD决定成肌细胞的命运,MyoD1蛋白持续高表达特点表明MyoD1不仅诱导骨骼肌特异性基因的表达,而且可能参与诱导多核肌管的伸长和融合。 "
| [1] Chen G, Tang Q, Yu S, et al. The biological function of Bmal1 in skeleton development and disorders. Life Sci. 2020;3:117636. [2] Shimizu T, Watanabe K, Anayama N, et al. Effect of lipopolysaccharide on circadian Clock genes Per2 and Bmal1 in mouse ovary. J Physiol Sci. 2017;67(5):623-628. [3] Yoshida K, Nakai A, Kaneshiro K, et al.TNF-a induces expression of the circadian Clock gene Bmal1 via dual calcium-dependent pathways in rheumatoid synovial cells. Biochem Biophys Res Commun. 2018;495(2):1675-1680. [4] Mayeuf-Louchart A, Staels B, Duez H. Skeletal muscle functions around the Clock. Diabetes Obes Metab. 2015;17 Suppl 1:39-46. [5] Gibbs JE, Ray DW. The role of the circadian Clock in rheumatoid arthritis. Arthritis Res Ther. 2013;15(1):205. [6] Wang Y, Pati P, Xu Y, et al. Endotoxin Disrupts Circadian Rhythms in Macrophages via Reactive Oxygen Species. PLoS One. 2016;11(5): e0155075. [7] Meng ZX, Gong J, Chen Z, et al. Glucose Sensing by Skeletal Myocytes Couples Nutrient Signaling to Systemic Homeostasis.Mol Cell. 2017;66(3):332-344. [8] Hodge BA, Zhang X, Gutierrez-Monreal MA, et al. MYOD1 functions as a Clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. Elife. 2019;8:e43017. [9] Wang C, Liu W, Nie Y, et al. Loss of MyoD promotes fate transdifferentiation of myoblasts into brown adipocytes. EBioMedicine. 2017;16:212-223. [10] Yoo YM, Jung EM, Jeung EB. Rapamycin-induced autophagy decreases Myf5 and MyoD proteins in C2C12 myoblast cells. Toxicol In Vitro. 2019;58:132-141. [11] Tu C, Bu Y, Vujcic M, et al. Ion Current-based Proteomic Profiling in Understanding the Inhibitory Effect of Tumor Necrosis Factor Alpha on Myogenic Differentiation. J Proteome Res. 2016;15(9):3147-3157. [12] Chatterjee S, Ma K. Circadian Clock regulation of skeletal muscle growth and repair. F1000Res. 2016;5:1549. [13] Riley LA, Esser KA. The Role of the Molecular Clock in Skeletal Muscle and What It Is Teaching Us About Muscle-Bone Crosstalk. Curr Osteoporos Rep. 2017;15(3):222-230. [14] Aoyama S, Shibata S. The Role of Circadian Rhythms in Muscular and Osseous Physiology and Their Regulation by Nutrition and Exercise.Front Neurosci. 2017;11:63. [15] 闫银弟,罗旭光,杨艳萍,等.钟基因在不同胎龄小鼠胚胎和孕鼠的表达规律[J].中国组织工程研究,2019,23(1):96-102. [16] Chatterjee S, Yin H, Nam D, et al. Brain and muscle Arnt-like 1 promotes skeletal muscle regeneration through satellite cell expansion.Exp Cell Res. 2015;331(1):200-210. [17] Chatterjee S, Yin H, Li W, et al. The Nuclear Receptor and Clock Repressor Rev-erbα Suppresses Myogenesis. Sci Rep. 2019;9(1): 4585. [18] Samant SA, Kanwal A, Pillai VB, et al. The histone deacetylase SIRT6 blocks myostatin expression and development of muscle atrophy. Sci Rep. 2017;7(1):11877. [19] 闫银弟,罗旭光,杨艳萍,等.骨骼肌昼夜节律分子钟机制的研究进展[J].实用医学杂志,2018,34(7):1213-1215. [20] Romagnoli C, Zonefrati R, Sharma P, et al. Characterization of Skeletal Muscle Endocrine Control in an In Vitro Model of Myogenesis.Calcif Tissue Int. 2020;107(1):18-30. [21] ROBINSON I, REDDY AB. Molecular mechanisms of the circadian Clock work in mammals. FEBS Lett. 2014;588(15):2477-2483. [22] Armand AS, Bourajjaj M, Martinez-Martinez S, et al. CooPerative synergy between NFAT and MyoD regulates myogenin expression and myogenesis. J Biol Chem. 2008;283(43):29004-29010. [23] de la Serna IL, Ohkawa Y, Berkes CA, et al. MyoD Targets Chromatin Remodeling Complexes to the Myogenin Locus Prior to Forming a Stable DNA-Bound Complex. Mol Cell Biol. 2005;25(10): 3997-4009. [24] Ganassi M, Badodi S, Ortuste Quiroga HP, et al. Myogenin promotes myocyte fusion to balance fibre number and size. Nat Commun. 2018;9(1):4232. [25] Higashioka K, Koizumi N, Sakurai H, et al. Myogenic differentiation from MYOGENIN-mutated human iPS cells by CRISPR/Cas9.Stem Cells Int. 2017;2017:9210494. [26] Himeda CL, Ranish JA, Hauschka SD. Quantitative proteomicidentification of MAZ as a transcriptional regulator of muscle-specific genes in skeletal and cardiac myocytes. Mol Cell Biol. 2008;28(20):6521-6535. [27] Shavlakadze T, Anwari T, Soffe Z, et al. Impact of fasting on the rhythmic expression of myogenic and metabolic factors in skeletal muscle of adult mice. Am J Physiol Cell Physiol. 2013;305(1):C26-C35. [28] Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses.Nat Rev Genet. 2012;13(4):227-232. [29] Sabari BR, Dall’Agnese A, Boija A, et al. Coactivator condensation at suPer-enhancers links phase separation and gene control. Science. 2018;361(6400).pii: eaar3958. [30] Boeynaems S, Alberti S, Fawzi NL, et al. Protein phase separation: A new phase in cell biology. Trends Cell Biol. 2018;28(6):420-435. [31] Leong I. Muscle circadian Clock regulates lipid storage.Nat Rev Endocrinol. 2018;14(10):563. [32] Vitale JA, Bonato M, La Torre A, et al. The Role of the Molecular Clock in Promoting Skeletal Muscle Growth and Protecting against Sarcopenia. Int J Mol Sci. 2019;20(17).pii: E4318. [33] Qiao M, Huang J, Wu H, et al. Molecular characterization, transcriptional regulation and association analysis with carcass traits of porcine TCAP gene. Gene. 2014;538(2):273-279. [34] Zhang X, Patel SP, McCarthy JJ, et al. A non-canonical E-box within the MyoD core enhancer is necessary for circadian expression in skeletal muscle. Nucleic Acids Res. 2012;40(8):3419-3430. |
| [1] | Li Jing, Xie Jianshan, Cui Huilin, Cao Ximei, Yang Yanping, Li Hairong. Expression and localization of diacylglycerol kinase zeta and protein kinase C beta II in mouse back skin with different coat colors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1196-1200. |
| [2] | Chen Jiming, Wu Xiaojing, Liu Tianfeng, Chen Haicong, Huang Chengshuo. Effects of silymarin on liver injury and bone metabolism induced by carbon tetrachloride in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1224-1228. |
| [3] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [4] | Wang Feng, Zhou Liyu, Saijilafu, Qi Shibin, Ma Yanxia, Wei Shanwen. CaMKII-Smad1 promotes axonal regeneration of peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1064-1068. |
| [5] | Zhang Mi, Wu Saixuan, Dong Ming, Lu Ying, Niu Weidong. Expression of interleukin-24 in a mouse model of periapical periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 679-684. |
| [6] | Xie Yang, Zhang Shujiang, Liu Menglan, Luo Ying, Yang Yang, Li Zuoxiao. Mechanism by which rapamycin protects spinal cord neurons in experimental autoimmune encephalomyelitis mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 695-700. |
| [7] | Yu Langbo, Qing Mingsong, Zhao Chuntao, Peng Jiachen. Hot issues in clinical application of dynamic contrast-enhanced magnetic resonance imaging in orthopedics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 449-455. |
| [8] | Bai Shengchao, Gao Yang, Wang Bo, Li Junping, Wang Ruiyuan. Dynamic changes of mitochondrial function of the skeletal muscle after acupuncture intervention in rats with heavy load exercise-induced injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3648-3653. |
| [9] | Xie Yang, Lü Zhiyu, Zhang Shujiang, Long Ting, Li Zuoxiao. Effects of recombinant adeno-associated virus mediated nerve growth factor gene transfection on oligodendrocyte apoptosis and myelination in experimental autoimmune encephalomyelitis mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3678-3683. |
| [10] | Wang Zhen, Lin Haiqi, He Fei, Lin Wentao. Exercise activates skeletal muscle satellite cells: exercise prevention and treatment for age-related sarcopenia and muscle injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3752-3759. |
| [11] | Li Shang, Huang Xiang, Chen Ming, Lei Mingxing, Cheng Shi, Zhang Licheng, Yin Pengbin, Tang Peifu. Semaphorin 3A is expected to be a new target for the repair of skeletal muscle injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3232-3238. |
| [12] | Li Zhen, Huang Yonghui, Sun Jifu, Sun Haitao. Role and mechanism of focal adhesion kinase in inducing osteogenic differentiation of mouse embryonic fibroblasts cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 165-171. |
| [13] | Geng Bin, Xia Yayi. Involvement of ERK5 signaling pathway in osteoporosis development in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 178-185. |
| [14] | Li Xiaoqun, Xu Kaihang, Ji Fang. Corylin inhibits osteoclastogenesis and attenuates postmenopausal osteoporosis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 186-190. |
| [15] | Liu Xing, Wei Xiaohan, Deng Jie, Li Zhongming . Preparing a blunt contusion model of rabbit skeletal muscle under different blow strengths [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 196-200. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||