Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (35): 5619-5624.doi: 10.3969/j.issn.2095-4344.2921
Previous Articles Next Articles
Autophagy has an early protective effect against fibroblast injury induced by advanced glycation end products
Han Yanfu1, Tao Ran2, Sun Tianjun3
- 1Department of Plastic Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China; 2Department of Plastic Surgery, First Medical Center, General Hospital of Chinese PLA,Beijing 100853, China; 3Department of Burn and Plastic Surgery, Fourth Medical Center, General Hospital of Chinese PLA, Beijing 100048, China
-
Received:2020-02-24Revised:2020-02-29Accepted:2020-03-30Online:2020-12-18Published:2020-10-16 -
About author:Han Yanfu, MD, Associate chief physician, Department of Plastic Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China -
Supported by:2020 Military Medical Science and Technology Project for Youth Training and Cultivation, No. 20QNPY097
CLC Number:
Cite this article
Han Yanfu, Tao Ran, Sun Tianjun. Autophagy has an early protective effect against fibroblast injury induced by advanced glycation end products[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(35): 5619-5624.
share this article
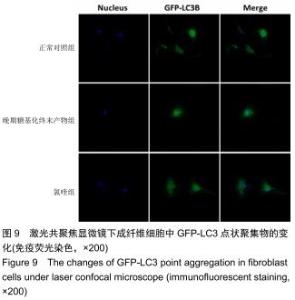
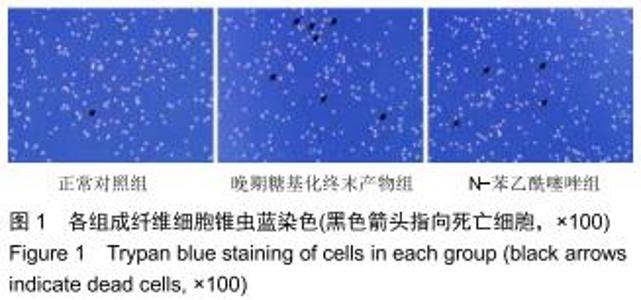
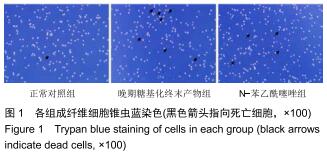
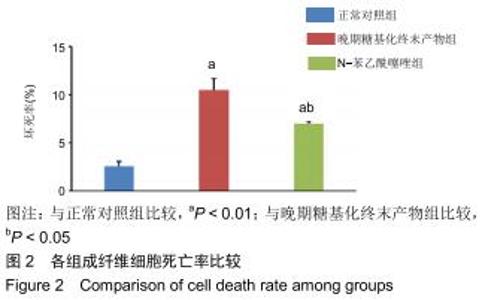
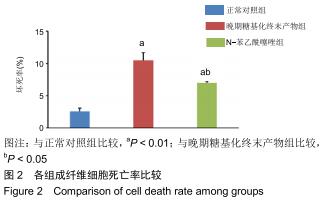
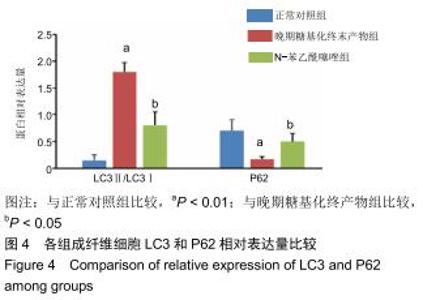
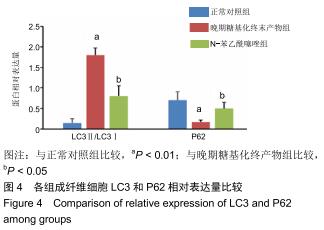
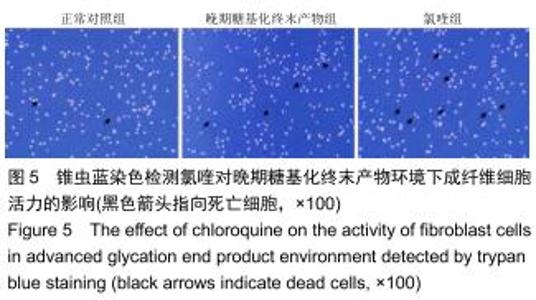
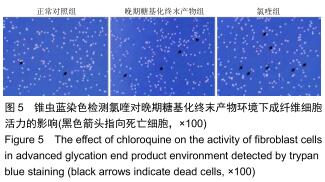
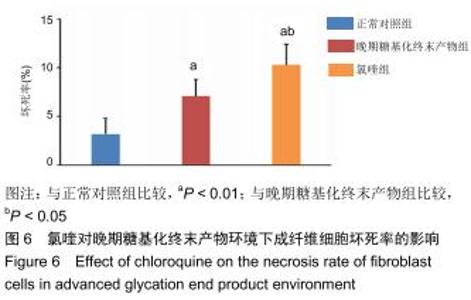
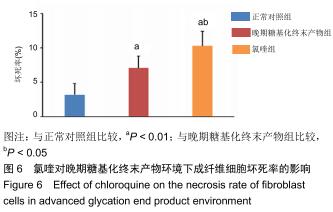
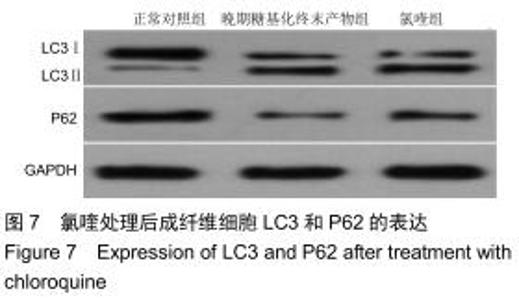
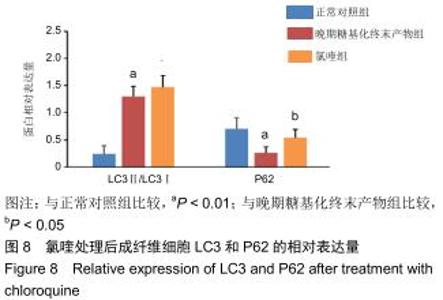
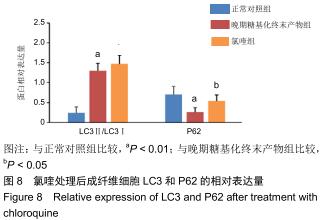
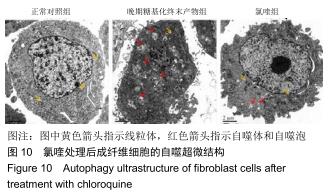
免疫荧光实验结果显示,与正常对照组比较,晚期糖基化终末产物组细胞中LC3绿色荧光点状聚集物增加,表明晚期糖基化终末产物处理可以诱导形成大量LC3Ⅱ标记的自噬体;晚期糖基化终末产物联合氯喹共同处理后,由于氯喹抑制自噬的机制是通过抑制自噬体与溶酶体的融合过程,因此氯喹可以抑制自噬完成,但会造成LC3Ⅱ积累,导致细胞中点状聚集物增加,见图9。透射电镜观察结果显示,正常对照组可见完整的细胞核及核膜,线粒体丰富,形态清晰;晚期糖基化终末产物组可见数目较多的自噬小体及内含物被降解后遗留的空泡和髓样小体结构,自噬程度明显增加;氯喹组细胞中亦可观察到自噬体结构,因氯喹抑制自噬体与溶酶体的融合过程,细胞中自噬体结构和空泡状结构比晚期糖基化终末产物组少,见图10。 "
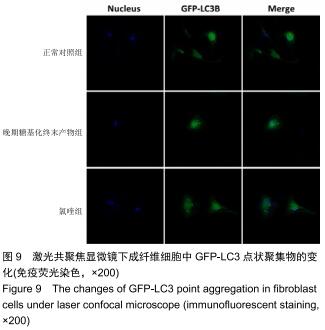
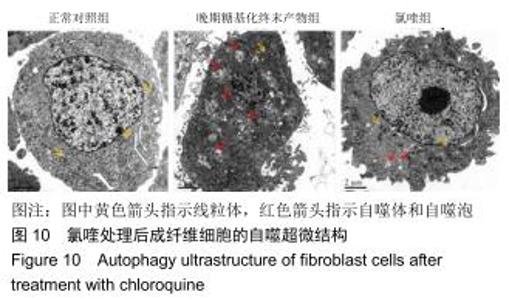
免疫荧光实验结果显示,与正常对照组比较,晚期糖基化终末产物组细胞中LC3绿色荧光点状聚集物增加,表明晚期糖基化终末产物处理可以诱导形成大量LC3Ⅱ标记的自噬体;晚期糖基化终末产物联合氯喹共同处理后,由于氯喹抑制自噬的机制是通过抑制自噬体与溶酶体的融合过程,因此氯喹可以抑制自噬完成,但会造成LC3Ⅱ积累,导致细胞中点状聚集物增加,见图9。透射电镜观察结果显示,正常对照组可见完整的细胞核及核膜,线粒体丰富,形态清晰;晚期糖基化终末产物组可见数目较多的自噬小体及内含物被降解后遗留的空泡和髓样小体结构,自噬程度明显增加;氯喹组细胞中亦可观察到自噬体结构,因氯喹抑制自噬体与溶酶体的融合过程,细胞中自噬体结构和空泡状结构比晚期糖基化终末产物组少,见图10。 "
|
[1] AJITH TA, VINODKUMAR P. Advanced Glycation End Products: Association with the Pathogenesis of Diseases and the Current Therapeutic Advances. Curr Clin Pharmacol. 2016;11(2):118-127.
[2] 陆树良,青春,谢挺,等.糖尿病皮肤“隐性损害”的机制研究[J].中华创伤杂志,2004,20(8):468-473.
[3] VERMA N, MANNA SK. Advanced Glycation End Products (AGE) Potently Induce Autophagy through Activation of RAF Protein Kinase and Nuclear Factor κB (NF-κB). J Biol Chem. 2016;291(3): 1481-1491.
[4] YU Y, WANG L, DELGUSTE F, et al. Advanced glycation end products receptor RAGE controls myocardial dysfunction and oxidative stress in high-fat fed mice by sustaining mitochondrial dynamics and autophagy-lysosome pathway. Free Radic Biol Med. 2017;112:397-410.
[5] VERMA N, MANNA SK. Advanced glycation end products (AGE) potentiates cell death in p53 negative cells via upregulaion of NF-kappa B and impairment of autophagy. J Cell Physiol. 2017; 232(12):3598-3610.
[6] PARZYCH KR, KLIONSKY DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460-473.
[7] LU K, PSAKHYE I, JENTSCH S. Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell. 2014;158(3):549-563.
[8] LIM J, LACHENMAYER ML, WU S, et al. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 2015;11(2):e1004987.
[9] 杨桂然,王福科,李彦林.成纤维细胞的生物学特性及分化潜能[J].中国组织工程研究,2020,24(13):2114-2119.
[10] NAZARUK J, BORZYM-KLUCZYK M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem Rev. 2015;14(4):675-690.
[11] SHAIKH-KADER A, HOURELD NN, RAJENDRAN NK, et al. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem Funct. 2019;37(6):432-442.
[12] 赵峻,马翅,杨娜娜,等.晚期糖基化终末产物致软骨细胞炎性反应的潜在机制[J].中国组织工程研究,2018,22(24): 3786-3791.
[13] DAI J, CHEN H, CHAI Y. Advanced Glycation End Products (AGEs) Induce Apoptosis of Fibroblasts by Activation of NLRP3 Inflammasome via Reactive Oxygen Species (ROS) Signaling Pathway. Med Sci Monit. 2019;25:7499-7508.
[14] MARTINS IJ. Human Survival and Immune Mediated Mitophagy in Neuroplasticity Disorders. Neural Regen Res. 2019;14(4):735.
[15] REGGIORI F, KLIONSKY DJ. Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol. 2005;17(4):415-422.
[16] XIE Z, KLIONSKY DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102-1109.
[17] TAKAHASHI A, TAKABATAKE Y, KIMURA T, et al. Autophagy Inhibits the Accumulation of Advanced Glycation End Products by Promoting Lysosomal Biogenesis and Function in the Kidney Proximal Tubules. Diabetes. 2017;66(5):1359-1372.
[18] LI M, LIU DW. Advances in the research of effects of regulation of cell autophagy on wound healing. Zhonghua Shao Shang Za Zhi. 2017;33(10):625-628.
[19] ZENG T, WANG X, WANG W, et al. Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin Sci (Lond). 2019;133(9): CS20190008.
[20] RABIEE MOTMAEN S, TAVAKOL S, JOGHATAEI MT, et al. Acidic pH derived from cancer cells as a double-edged knife modulates wound healing through DNA repair genes and autophagy. Int Wound J. 2020;17(1):137-148.
[21] CHEN W, WU Y, LI L, et al. Adenosine accelerates the healing of diabetic ischemic ulcers by improving autophagy of endothelial progenitor cells grown on a biomaterial. Sci Rep. 2015;5:11594.
[22] ZHENG A, MA H, LIU X, et al. Effects of Moist Exposed Burn Therapy and Ointment (MEBT/MEBO) on the autophagy mTOR signalling pathway in diabetic ulcer wounds. Pharm Biol. 2020; 58(1):124-130.
[23] 王朝俊,陈铖,张莹,等.晚期糖基化终末产物(AGEs)对人软骨细胞自噬活性的影响研究[J].航空航天医学杂志,2018,29(1):51-53.
[24] KOCATURK NM, AKKOC Y, KIG C, et al. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019; 134:116-137.
[25] COOPER ME, THALLAS V, FORBES J, et al. The cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced glycation end-product accumulation. Diabetologia. 2000;43(5):660-664.
[26] YAMAGISHI S. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Res. 2012;15(6):564-572.
[27] BRADKE BS, VASHISHTH D. N-phenacylthiazolium bromide reduces bone fragility induced by nonenzymatic glycation. PLoS One. 2014;9(7):e103199.
[28] MATHEW R, KARP CM, BEAUDOIN B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062-1075.
[29] CHEN S, ZHOU L, ZHANG Y, et al. Targeting SQSTM1/p62 induces cargo loading failure and converts autophagy to apoptosis via NBK/Bik. Mol Cell Biol. 2014;34(18):3435-3449.
[30] VEGLIANTE R, DESIDERI E, DI LEO L, et al. Dehydroepiandrosterone triggers autophagic cell death in human hepatoma cell line HepG2 via JNK-mediated p62/SQSTM1 expression. Carcinogenesis. 2016;37(3):233-244.
[31] XU F, LI J, NI W, et al. Peroxisome proliferator-activated receptor-γ agonist 15d-prostaglandin J2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS One. 2013;8(1):e55080.
[32] WU D, WANG H, TENG T, et al. Hydrogen sulfide and autophagy: A double edged sword. Pharmacol Res. 2018;131:120-127.
[33] LI ZY, WU YF, XU XC, et al. Autophagy as a double-edged sword in pulmonary epithelial injury: a review and perspective. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L207-L217. [34] HAN Y, SUN T, TAO R, et al. Clinical application prospect of umbilical cord-derived mesenchymal stem cells on clearance of advanced glycation end products through autophagy on diabetic wound. Eur J Med Res. 2017;22(1):11. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [4] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [5] | Wu Xun, Meng Juanhong, Zhang Jianyun, Wang Liang. Concentrated growth factors in the repair of a full-thickness condylar cartilage defect in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1166-1171. |
| [6] | Liu Zhichao, Zhang Fan, Sun Qi, Kang Xiaole, Yuan Qiaomei, Liu Genzhe, Chen Jiang. Morphology and activity of human nucleus pulposus cells under different hydrostatic pressures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1172-1176. |
| [7] | Li Jing, Xie Jianshan, Cui Huilin, Cao Ximei, Yang Yanping, Li Hairong. Expression and localization of diacylglycerol kinase zeta and protein kinase C beta II in mouse back skin with different coat colors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1196-1200. |
| [8] | Li Jiacheng, Liang Xuezhen, Liu Jinbao, Xu Bo, Li Gang. Differential mRNA expression profile and competitive endogenous RNA regulatory network in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1212-1217. |
| [9] | Geng Qiudong, Ge Haiya, Wang Heming, Li Nan. Role and mechanism of Guilu Erxianjiao in treatment of osteoarthritis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1229-1236. |
| [10] | Tan Jingyu, Liu Haiwen. Genome-wide identification, classification and phylogenetic analysis of Fasciclin gene family for osteoblast specific factor 2 [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1243-1248. |
| [11] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [12] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [13] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [14] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [15] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||