Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (33): 5339-5347.doi: 10.3969/j.issn.2095-4344.2017.33.015
Previous Articles Next Articles
Cartilage repair using induced pluripotent stem cell derived chondrocytes in osteoarthriti
Wu Zhang-song1, Zhu Hong-xia2, Luo Zhi-qiang1, Wu Xiao-min1, Lin Guang-yao1, Zhang Ming-yu1, Gu Hong-sheng2,Zhu Yan-xia1
- 1Shenzhen Key Laboratory for Anti-ageing and Regenerative Medicine, Health Science Center, Shenzhen University, Shenzhen 518060, Guangdong Province, China; 2Department of Spinal Surgery, Xiaogan Maternity & Child Healthcare Hospital, Xiaogan 432100, Guangdong Province, China
-
Revised:2017-06-07Online:2017-11-28Published:2017-12-01 -
Contact:Zhu Yan-xia, M.D., Associate professor, Shenzhen Key Laboratory for Anti-ageing and Regenerative Medicine, Health Science Center, Shenzhen University, Shenzhen 518060, Guangdong Province, China -
About author:Wu Zhang-song, Shenzhen Key Laboratory for Anti-ageing and Regenerative Medicine, Health Science Center, Shenzhen University, Shenzhen 518060, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81301597; Shenzhen Emerging Strategic Industrial Development Special Funds, No. JCYJ20130326110154156, JCYJ20150525092940984, and CYJ20160422090807181
CLC Number:
Cite this article
Wu Zhang-song, Zhu Hong-xia, Luo Zhi-qiang, Wu Xiao-min, Lin Guang-yao, Zhang Ming-yu, Gu Hong-sheng,Zhu Yan-xia. Cartilage repair using induced pluripotent stem cell derived chondrocytes in osteoarthriti[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(33): 5339-5347.
share this article
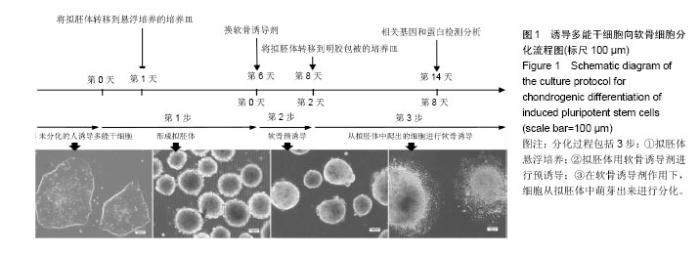
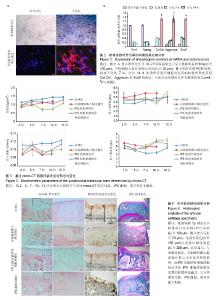
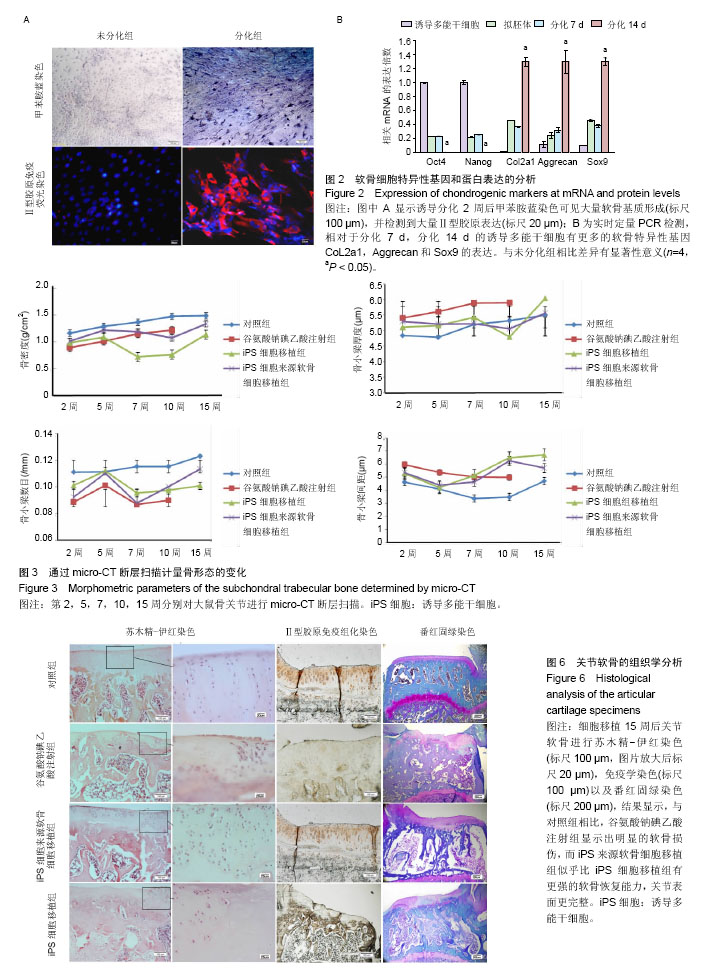
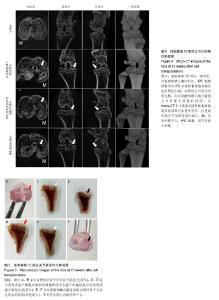
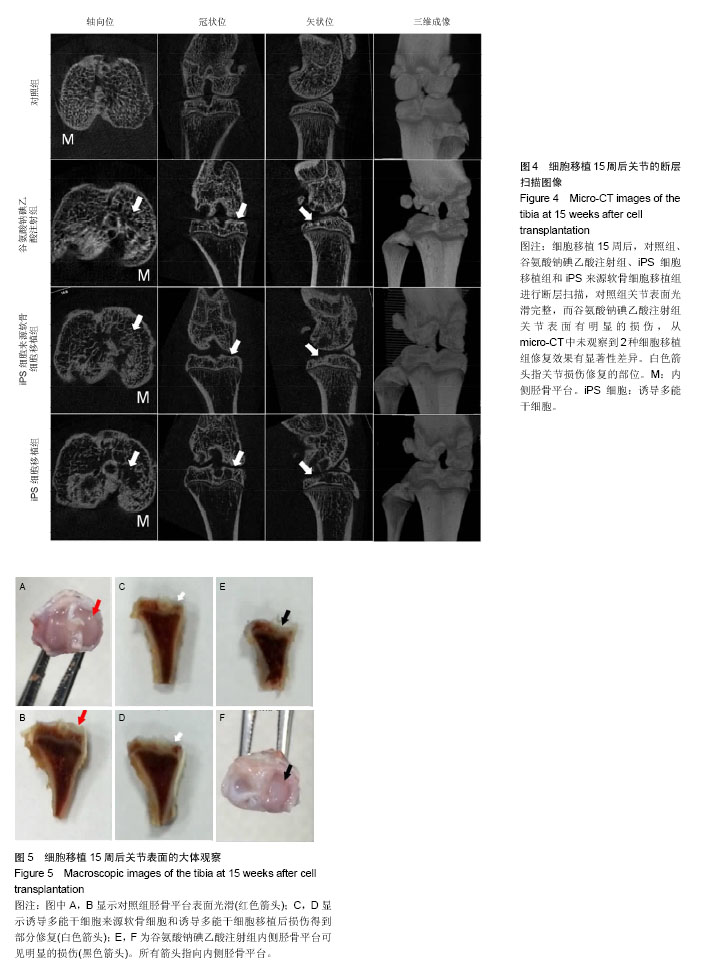
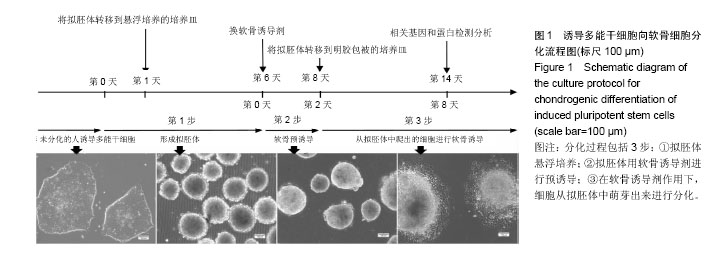
2.1 iPS细胞形态学改变 在分化之前,iPS细胞呈单层,细胞较小,呈纤维状,当细胞在甲基纤维素上培养后,细胞呈克隆样生长,选择大小相似的拟胚体进行诱导实验。在将拟胚体细胞贴壁培养之前,在拟胚体细胞悬液中加入软骨诱导剂进行预诱导。在预诱导过程中,拟胚体表现出良好的细胞形态,很少有内部细胞坏死。将拟胚体转移到明胶包被的培养皿中,用软骨诱导剂培养一两周后,会有软骨样的较大球形细胞从拟胚体上萌芽出来,见图1。 2.2 免疫化学分析 诱导分化2周后,整个带有萌芽细胞的拟胚体都被甲苯胺蓝染上较深蓝色(图2A),表明有软骨外基质形成。而未分化组并没有明显的蓝色出现。为了确定iPS细胞的软骨分化能力,检测到分化的拟胚体有明显的Ⅱ型胶原的表达(红色荧光,图2A)。 为了进一步确认软骨细胞分化程度,在分化的第7天和第14天,分别用荧光定量PCR对软骨特异性基因和干性相关基因进行检测,结果发现,诱导2周后Col2a1,Aggrecan和Sox9的表达量显著升高。与未分化的iPS细胞相比,分化后细胞Oct4和Nanog的表达量显著性下降。而未分化的拟胚体和分化了7 d的iPS细胞这两种基因表达水平差异无显著性意义(图2B)。 2.3 骨和软骨下骨定量变化 细胞移植后2,5,7,10,15周,采用micro-CT观察骨密度、骨小梁厚度(Tb.Th)、骨小梁数目(Tb.N)和骨小梁间距(Tb.Sp)变化,随着时间的推移,谷氨酸钠碘乙酸注射组和对照组大鼠胫骨软骨下软骨的骨密度、骨小梁厚度和骨小梁数目明显上升,15周后,两组胫骨骨小梁间距与第2周相比,明显减小(图3)。 细胞移植后2周,与对照组相比,谷氨酸钠碘乙酸注射组骨密度和骨小梁数目均明显下降。细胞移植后5周,骨密度值略有上升,而第7周可能已经出现骨质疏松症状,骨密度明显较低。但是第10周骨密度值又显著上升,并且与对照组值接近,这可能是软骨内骨化造成的。细胞移植后第5周,骨小梁数目值突然上升,软骨下骨的骨小梁断裂可能是导致骨小梁数目上升的原因。移植未分化iPS细胞组和移植分化后iPS细胞组骨密度和骨小梁数目没有显著性差异。到第15周,两个细胞移植组和对照组胫骨骨小梁数目没有明显差异。 细胞移植后第7周,骨小梁厚度值下降至与对照组接近。细胞移植后第5周,尽管骨小梁间距值略有升高,但在接下来几周时间里迅速下降。15周之后,移植软骨分化后iPS细胞组与移植未分化的iPS细胞组相比,其骨密度、骨小梁厚度、骨小梁数目和骨小梁间距值均接近于对照组。 2.4 软骨下骨形态学改变 谷氨酸钠碘乙酸注射组所有大鼠都表现出软骨下骨的病理学改变,然而在整个研究过程中(15周)对照组胫骨的软骨下骨没有任何骨关节炎样变化。随着时间的推移,对谷氨酸钠碘乙酸注射组关节形态进行追踪,发现注射谷氨酸钠碘乙酸后的第15周胫骨软骨下骨出现硬化,特别是胫骨内髁部位(图4)。注射谷氨酸钠碘乙酸的胫骨软骨终板在核心区域出现断裂,组织学分析也可见这些断裂的出现。从micro-CT图像可以观察到,谷氨酸钠碘乙酸注射15周后,其膝盖中心胫骨软骨下骨出现空隙,这些空隙通过组织切片观察被确认为是囊肿。通过对所有膝盖的3个空间区域进行分析发现,谷氨酸钠碘乙酸注射组与对照组相比,其胫骨软骨下骨终板的空隙率显著上升(P < 0.01)。谷氨酸钠碘乙酸注射组膝关节三维重构图显示胫骨软骨下骨板出现侵蚀和点蚀,其内侧胫骨平台更严重,而对照组膝关节软骨下骨板表面完整。细胞移植后第15周观察到移植2种细胞的膝关节没有表现出明显形态学差异。但与谷氨酸钠碘乙酸注射组相比,细胞移植组胫骨软骨下骨没有观察到断裂或者空隙,只是胫骨软骨下骨终板的孔隙率略有下降(图4)。 2.5 大体观察 注射谷氨酸钠碘乙酸的关节都表现出软骨损伤,软骨终板很容易从关节部位剥落,但谷氨酸钠碘乙酸注射后再进行细胞移植,软骨终板会紧紧地贴合在关节部位,很难剥落。谷氨酸钠碘乙酸注射组和细胞移植组膝关节胫骨软骨表现出褪色的局灶性病变,表明软骨出现退行性变(图5,黑色箭头),该现象在内侧胫骨平台的中央部位表现得尤为明显,而边缘部位的软骨退变较轻。对照组胫骨中间和边缘区域均未表现出软骨损伤(图5,红色箭头)。移植iPS细胞和iPS来源软骨细胞后,软骨损伤得到部分修复。移植iPS来源的软骨细胞与移植iPS细胞相比,表现出更强的修复能力(图5,白色箭头)。 2.6 组织学分析结果 对照组的关节软骨表现出典型的四层完整细胞层,包括表层、过渡层、径向深层和钙化区域,细胞外基质的主要成分是均匀分布的蛋白多糖(图6)。然而,所有注射谷氨酸钠碘乙酸的关节软骨都表现退化。在第15周时,软骨下骨终板出现软骨损伤,在中心区域出现裂痕。组织切片出现骨关节炎样特征,例如表面不连续性,蛋白多糖含量下降,软骨细胞减少以及软骨下骨硬化。移植iPS细胞来源软骨细胞后,与谷氨酸钠碘乙酸注射组相比,软骨表面变得连续,蛋白多糖含量上升,出现了软骨细胞增殖,表明正在修复软骨。但是iPS细胞移植组的效果并没有这样明显(图6)。 对新的软骨组织进行Ⅱ型胶原免疫染色分析,结果表明iPS细胞来源软骨细胞可修复软骨组织(图6)。与对照组软骨相比,谷氨酸钠碘乙酸注射组的软骨表达很少的胶原蛋白,当注射iPS细胞和iPS细胞来源软骨细胞后,Ⅱ型胶原蛋白明显上升,移植iPS细胞来源软骨细胞组比移植iPS细胞组可表达更多的Ⅱ型胶原蛋白,表明iPS细胞来源软骨细胞比iPS细胞具有更好的软骨修复效果,但仍然无法达到对照组水平。 番红-固绿复染的胫骨软骨切片图像如图6所示。谷氨酸钠碘乙酸注射组软骨出现大量的蛋白多糖流失,对照组并没有观察到此现象。移植iPS细胞之后,中间的胫骨平台变厚出现局部退化,骨头表面仍然有斜裂现象,软骨下骨出现骨吸收现象,此外可观察到软骨下骨重建及变厚现象。但总体来说iPS细胞来源软骨细胞组的修复效率要明显高于iPS细胞组。"
| [1] World Health Organisation Scientific Group. The Burden of Musculoskeletal Conditions at the Start of the New Millennium. Geneva: World Health Organization,2003.[2] Hunter DJ. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol. 2011; 7(1):13-22.[3] Lohmander LS, Roos EM. Clinical update: treating osteoarthritis. Lancet. 2007;370(9605):2082-2084.[4] Matricali GA, Dereymaeker GP, Luyten FP. Donor site morbidity after articular cartilage repair procedures: a review. Acta Orthop Belg. 2010;76(5):669-674.[5] Kretlow JD, Jin YQ, Liu W, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60.[6] Hubbard JJ, Sullivan SK, Mills JA, et al. Efficient iPS cell generation from blood using episomes and HDAC inhibitors. J Vis Exp. 2014;(92):e52009.[7] Trokovic R, Weltner J, Nishimura K, et al. Advanced feeder-free generation of induced pluripotent stem cells directly from blood cells. Stem Cells Transl Med. 2014;3(12): 1402-1409.[8] Saito T, Yano F, Mori D, et al. Generation of Col2a1-EGFP iPS cells for monitoring chondrogenic differentiation. PLoS One. 2013;8(9):e74137.[9] Liu Z, Tang Y, Lü S, et al. The tumourigenicity of iPS cells and their differentiated derivates. J Cell Mol Med. 2013;17(6): 782-791.[10] Teramura T, Onodera Y, Mihara T, et al. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cell Reprogram. 2010;12(3):249-261.[11] Kim MJ, Son MJ, Son MY, et al. Generation of human induced pluripotent stem cells from osteoarthritis patient-derived synovial cells.Arthritis Rheum. 2011;63(10):3010-3021.[12] Koyama N, Miura M, Nakao K, et al. Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem Cells Dev. 2013;22(1):102-113.[13] Giuliani N, Lisignoli G, Magnani M, et al. New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cells Int. 2013;2013:312501.[14] Illich DJ, Demir N, Stojkovi? M, et al. Concise review: induced pluripotent stem cells and lineage reprogramming: prospects for bone regeneration. Stem Cells. 2011;29(4):555-563.[15] Giovannini S, Diaz-Romero J, Aigner T, et al. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater. 2010;20:245-259.[16] Diecke S, Jung SM, Lee J, et al. Recent technological updates and clinical applications of induced pluripotent stem cells. Korean J Intern Med. 2014;29(5):547-557.[17] Csobonyeiova M, Polak S, Koller J, et al. Induced pluripotent stem cells and their implication for regenerative medicine. Cell Tissue Bank. 2015;16(2):171-180.[18] Bigdeli N, Karlsson C, Strehl R, et al. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells. 2009;27(8):1812-1821.[19] Craft AM, Ahmed N, Rockel JS, et al. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development. 2013;140(12):2597-2610.[20] Vinatier C, Mrugala D, Jorgensen C, et al. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27(5):307-314.[21] Olee T, Grogan SP, Lotz MK, et al. Repair of cartilage defects in arthritic tissue with differentiated human embryonic stem cells. Tissue Eng Part A. 2014;20(3-4):683-692.[22] Hino K, Saito A, Kido M, et al. Master regulator for chondrogenesis, Sox9, regulates transcriptional activation of the endoplasmic reticulum stress transducer BBF2H7/ CREB3L2 in chondrocytes. J Biol Chem. 2014;289(20): 13810-13820.[23] Huh JE, Koh PS, Seo BK, et al. Mangiferin reduces the inhibition of chondrogenic differentiation by IL-1β in mesenchymal stem cells from subchondral bone and targets multiple aspects of the Smad and SOX9 pathways. Int J Mol Sci. 2014;15(9):16025-16042.[24] Yang W, Lee S, Jo YH, et al. Effects of natural cartilaginous extracellular matrix on chondrogenic potential for cartilage cell transplantation. Transplant Proc. 2014;46(4):1247-1250. [25] Medvedev SP, Grigor'eva EV, Shevchenko AI, et al. Human induced pluripotent stem cells derived from fetal neural stem cells successfully undergo directed differentiation into cartilage. Stem Cells Dev. 2011;20(6):1099-1112.[26] Im HJ, Kim JS, Li X, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62(10):2995-3005.[27] Ferreira-Gomes J, Adães S, Sousa RM, et al. Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat. Mol Pain. 2012;8:50.[28] Moon SJ, Jeong JH, Jhun JY, et al. Ursodeoxycholic Acid ameliorates pain severity and cartilage degeneration in monosodium iodoacetate-induced osteoarthritis in rats. Immune Netw. 2014;14(1):45-53.[29] Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):43-49. [30] Li B, Aspden RM. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997;56(4): 247-254.[31] Zhen G, Wen C, Jia X, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704-712.[32] Hubka KM, Dahlin RL, Meretoja VV, et al. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng Part B Rev. 2014;20(6): 641-654. |
| [1] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [2] | Zhang Jichao, Dong Yuefu, Mou Zhifang, Zhang Zhen, Li Bingyan, Xu Xiangjun, Li Jiayi, Ren Meng, Dong Wanpeng. Finite element analysis of biomechanical changes in the osteoarthritis knee joint in different gait flexion angles [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1357-1361. |
| [3] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [4] | Wang Baojuan, Zheng Shuguang, Zhang Qi, Li Tianyang. Miao medicine fumigation can delay extracellular matrix destruction in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1180-1186. |
| [5] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [6] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [7] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [8] | Liu Dongcheng, Zhao Jijun, Zhou Zihong, Wu Zhaofeng, Yu Yinghao, Chen Yuhao, Feng Dehong. Comparison of different reference methods for force line correction in open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 827-831. |
| [9] | Zhou Jianguo, Liu Shiwei, Yuan Changhong, Bi Shengrong, Yang Guoping, Hu Weiquan, Liu Hui, Qian Rui. Total knee arthroplasty with posterior cruciate ligament retaining prosthesis in the treatment of knee osteoarthritis with knee valgus deformity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 892-897. |
| [10] | He Junjun, Huang Zeling, Hong Zhenqiang. Interventional effect of Yanghe Decoction on synovial inflammation in a rabbit model of early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 694-699. |
| [11] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [12] | Xu Lei, Han Xiaoqiang, Zhang Jintao, Sun Haibiao. Hyaluronic acid around articular chondrocytes: production, transformation and function characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 768-773. |
| [13] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [14] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [15] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||