Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (32): 5197-5202.doi: 10.3969/j.issn.2095-4344.2017.32.019
Previous Articles Next Articles
Roles of modifications of chromatin and histone in osteogenesis
Zhang Wen-hai
- (Department of Orthopedics, Tianjin Hospital, Tianjin 300211, China)
-
Received:2017-06-10Online:2017-11-18Published:2017-11-15 -
About author:Zhang Wen-hai, Master, Associate chief physician, Department of Orthopedics, Tianjin Hospital, Tianjin 300211, China -
Supported by:the Natural Science Foundation of Tianjin, No. 11JCYBJC10200
CLC Number:
Cite this article
Zhang Wen-hai. Roles of modifications of chromatin and histone in osteogenesis[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(32): 5197-5202.
share this article
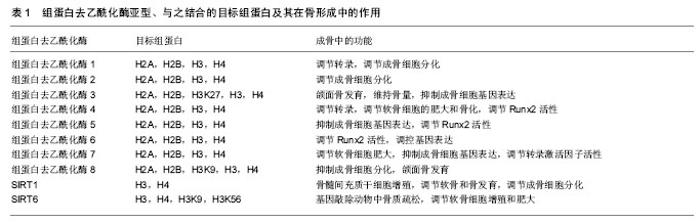
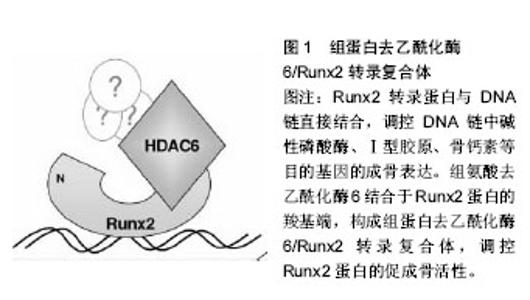
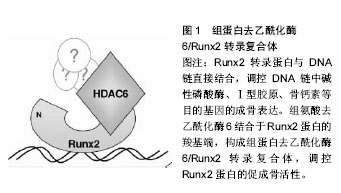
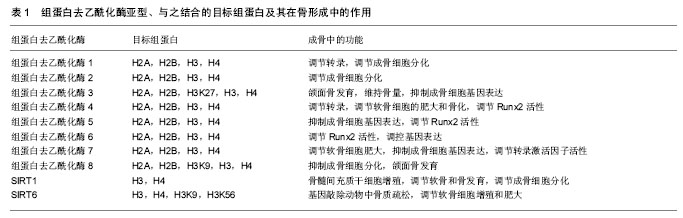
2.1 DNA甲基化 DNA甲基化能引起染色质的结构、DNA构象、DNA稳定性以及DNA与蛋白质相互作用方式的改变,从而控制基因表达。DNA甲基化为正常发育所必需。在哺乳动物中,胞嘧啶甲基化形成5-甲基胞嘧啶,这种DNA修饰呈现高度保守[5]。体细胞内胞嘧啶甲基化在很大程度上依赖于回文CpG二核苷酸结构。在卵母细胞、多能胚胎干细胞和成熟神经元中,并未观察到CpG甲基化现象[6-7]。DNA甲基化在细胞发育过程中受到动态调节,这种修饰有助于发育阶段细胞特异性表观遗传信号的传递,对基因表达影响深刻。接近70%的基因增强子区域和大量的转录起始区域(TSS)存在短的稠密CpG序列。这说明大量CpG岛内基因对甲基化反应敏感,但CpG岛常常并没有出现甲基化。在细胞正常发育阶段,CpG岛甲基化可以导致基因转录处在稳定的沉默状态。或者,CpG岛甲基化并不是基因沉默的启动事件,而是对基因长期不活跃的一种反应[8]。但在特定组织中,甲基化往往提示基因静止;甲基化状态改变也提示特定细胞生命活动得到活化或出现静止。从作用机制上讲,CpG甲基化可以直接抑制转录因子,或募集甲基结合蛋白,继而募集染色质修饰酶和复合体,使基因沉默。 2.2 DNA甲基化的生物调控 通常3种高度保守的酶协同作用,实现CpG甲基化:DNA甲基转移酶1、DNA甲基转移酶3A和DNA甲基转移酶3B。在细胞有丝分裂中,DNA甲基转移酶1/UHRF1识别模板链上的甲基化CpGs,并相应的将甲基基团投放在新生DNA上[9]。DNA甲基化状态能保证细胞分裂中大多数基因区域的表观遗传。但同时,甲基化也常受到加强、抑制,甚至被DNA甲基转移酶间接移除。例如,许多CpG岛增强子是非甲基化的。但在细胞发育或谱系提交中特异性增强子是甲基化的,从而实现基因沉默。在这一过程中,DNA甲基转移酶3A和DNA甲基转移酶3B识别与其他表观抑制结构(如组蛋白去乙酰化酶和甲基转移酶)相结合的增强子,移除H3K9和/或H3K27甲基,协同抑制基因活性[9]。CpG岛的甲基化状态也有利于调控复合体与特异蛋白区域相结合。UHRF1中的SRA域能识别蛋白中的半甲基化(mCG:GC)和甲基化CpG结合域(MBD)。如MECP2、MBD1、MBD2和MBD4能识别对称性的甲基化(mCG:GCm)CpG二分体。几种锌指CxxC结构域蛋白,包括KDM2a(Fbxl11)、KDM2b(Fbxl10)和MLL1,特异性识别非甲基化DNA(CG:GC),募集CpG岛的染色质修饰活动[10]。DNA双加氧酶的TET家族能启动CpG残基DNA的去甲基化。TET酶将5-甲基胞嘧啶依次转化为5-羟甲基胞嘧啶、5-甲酸基胞嘧啶和5-羧基胞嘧啶,实现核苷酸或碱基切除修复[11]。 2.3 骨内DNA甲基化 骨性关节炎是DNA甲基化在肌肉骨骼系统疾病研究中探索比较多的领域。随着骨性关节炎病情的进展,人类关节软骨细胞基质金属蛋白酶13和诱导型一氧化氮合成酶增强子的甲基化程度增加[12]。一些实验检测了特异性基因增强子的DNA甲基化水平,这些基因是成骨细胞分化的潜在推动剂。人类间充质干细胞向成骨细胞分化,引起多能干细胞基因或ERα信号通路相关基因的增强子甲基化水平提高[13-14]。在细胞成骨分化中,成骨细胞相关基因Bglap2(骨钙素)甲基化水平的改变,提示着细胞分化进程。在对MC-3T3-E1成骨细胞行同型半胱氨酸、白细胞介素6条件培养试验中,检测到DNA甲基转移酶1水平和随后的特异基因增强子的甲基化水平均有提高[15]。 2.4 组蛋白翻译后修饰 组蛋白有5种类型,包括H1、H2A、H2B、H3、H4。它富含带正电荷的碱性氨基酸(精氨酸和赖氨酸),能够同DNA中带负电荷的磷酸基团相互作用。它是一类小分子碱性蛋白质,并且组蛋白是已知蛋白质中最保守的蛋白质。组蛋白化学修饰发生在组蛋白N端尾部,尤其是组蛋白H3和H4的修饰促进染色质结构的变化。组蛋白尾部由20个氨基酸组成,并且从DNA转弯处的核小体间延伸出来。 真核细胞染色质形成核小体,核小体是多个组蛋白亚单位和DNA的复合体,它保护着DNA和表观遗传信息。组蛋白的翻译后修饰,是表观遗传调控的关键环节,影响谱系提交和基因表达。可复性的共价组蛋白修饰最常发生在化学结构不稳定的氨基酸残基(如赖氨酸、精氨酸、丝氨酸、苏氨酸、酪氨酸和组氨酸)的氨基端和羧基端,也可发生在核小体核的组蛋白反折或球状结构域内[16]。每个修饰后组蛋白残基都承载着特异性信息。例如,H3K4me3甲基化是广泛研究中的组蛋白修饰,它通常提示基因转录处于稳定或活跃状态。信息传递可通过多种机制,包括改变组蛋白间或组蛋白-DNA间的相互作用,募集能够影响组蛋白修饰和重构过程的结合因子[17]。修饰组蛋白甲基化的最常见位点包括:赖氨酸:H3K4、H3K9、H3K27、H3K36、H3K79和H4K20;精氨酸(R):H3R2、H3R8、H3R17、H3R26和H4R3。然而,位于组蛋白H1和核组蛋白H2A、H2B、H3和H4上的几种其他残基,会以不同的方式进行修饰。尽管组蛋白修饰表现各异,以此来提供特定基因的表达信息,但细胞内总的蛋白修饰展现出涉及个体细胞表观遗传状态的复合型组蛋白编码。由于特异性修饰会产生协同或拮抗效果,而且不同核小体或群落中不同细胞的修饰类型不同,因此这种编码信息变得愈加复杂。例如,胚胎干细胞的许多增强子同时有H3K4me3和H3K27me3标志,它们相互拮抗,这些标志分子与转录活化或抑制状态有关[18]。亚单位复合体中含有众多组蛋白修饰酶。修饰后的残基产生出与核小体相锚合的酶所必需的结合位点。例如,指南针样组蛋白修饰复合体能识别、去甲基化H3K27me3(通过UTX作用),同时甲基化H3K4(通过MLL3/4作用),乙酰化H3K9和H4K16(通过WDR5作用)[19]。 相对而言,组蛋白的甲基化修饰方式是最稳定的,所以最适合作为稳定的表观遗传信息。而乙酰化修饰具有较高的动态,另外还有其他不稳定的修饰方式,如磷酸化、腺苷酸化、泛素化、ADP核糖基化等等。这些修饰更为灵活地影响染色质的结构与功能,通过多种修饰方式的组合发挥其调控功能,所以有人称这些能被专门识别的修饰信息为组蛋白密码。这些组蛋白密码组合变化非常多,因此组蛋白共价修饰可能是更为精细的基因表达方式。另外,研究发现H2B的泛素化可以影响H3K4和H3K79的甲基化,这也提示了各种修饰间也存在着相互的关系。 2.5 组蛋白甲基化和乙酰化的调控 蛋白或蛋白复合体能调控组蛋白标志物,它们合成或移除特异性的共价修饰物,并被蛋白识别、结合,改变基因表达。这些蛋白可根据酶的功能特点分类,如组蛋白/赖氨酸去乙酰化酶、组蛋白/赖氨酸乙酰转移酶(HAT/KAT)、赖氨酸去甲基化酶(KDM);也可根据蛋白的结合活性分类,如溴区结构域是指结合乙酰化赖氨酸的蛋白,染色质域是指结合甲基化赖氨酸的蛋白。已经证实主要有3类酶能催化甲基基团结合到组蛋白赖氨酸的残基上:含蛋白的SET域、DOT1样蛋白、蛋白精氨酸N-甲基转移酶(PRMT)[20]。逆转赖氨酸残基甲基化的酶(去甲基化酶/赖氨酸去甲基化酶)大致可分为2类:胺氧化酶和含jumonjiC结构域铁依赖性双加氧酶。这些酶从酵母到人类高度保守,能使组蛋白或非组蛋白底物去甲基化。 赖氨酸残基乙酰化是将乙酰基基团通过赖氨酸乙酰转移酶(有时指组蛋白乙酰转移酶)转移到组蛋白残基上。该基团的去除由组蛋白去乙酰化酶完成。根据已发现的酶的同源性,赖氨酸乙酰转移酶可分为两类:GNAT(Gcn5)家族和MYST(Moz/Ybf2/Sas2/Tip60)家族。其他一些含有较少保守催化功能域的酶,如CBP/ p300(KAT3A/KAT3B),在真核细胞中具有重要功能[21]。有4类组蛋白去乙酰化酶:Ⅰ型和Ⅱ型分别与酵母scRpd3和scHda1密切相关;Ⅲ型指sirtuins,为NAD+依赖性,与酵母Sir2蛋白类似;Ⅳ型组蛋白去乙酰化酶只有组蛋白去乙酰化酶11一名成员[22]。除了组蛋白,许多赖氨酸乙酰转移酶和组蛋白去乙酰化酶能作用于细胞质和细胞核内的多种蛋白,提示它们在表观遗传和细胞生命过程中均具有重要功能[23]。 2.6 骨内组蛋白修饰 促进骨形成的组蛋白编码或表观遗传活动呈现相对非特异性。最近有学者研究了人类骨髓间充质干细胞和MC-3T3-E1前成骨细胞成骨分化中,组蛋白修饰在Runx2蛋白结合活动中的作用[24]。 促进骨形成的基本表观遗传修饰正在研究当中,大量研究致力于明确骨形成过程中组蛋白修饰活动的调控机制。成骨细胞或骨髓间充质干细胞模型中甲基转移酶和去甲基化酶功能已经直接获得评估。有学者通过基因敲除/转基因的互补试验,证实精氨酸甲基转移酶PRMT4(CARM1)通过Sox9甲基化在软骨内成骨和软骨细胞增殖中发挥作用[25]。人类Wolf-Hirschhorn综合征是包括颅面缺陷在内的骨发育和生长异常,WHSC1基因最初获得研究是源于它在这种疾病中的作用[26]。WHSC1基因也称为NSD2基因,编码KMT,后者能够甲基化H3K36。在大鼠模型中敲除此基因,会影响Runx2和P300的相互作用,严重影响骨发育。除此之外,NSD1蛋白是H3K36的特异性甲基转移酶,这种酶与Soto综合征有关,这种综合征包括巨头畸形和骨骼加速老化。PRC2相关H3K27甲基转移酶Ezh2(KMT6)直接抑制成骨过程,这可能是通过促进脂源性分化或阻断成骨基因表达实现[27-28]。但中央脊细胞向骨软骨源性前体细胞分化过程,还可能需要Ezh2参与。 有研究评估了去甲基化酶在成骨细胞呈递和分化中的作用。间充质细胞呈递中H3K27去甲基化酶UTX(KDM6A)高表达,使该细胞骨源性分化超过脂源性分化。Jmjd3(KDM6B)或Jmjd2B(KDM4B)表达降低,会分别增加H3K27或H3K9甲基化水平,进而抑制成骨分化,降低成骨基因表达[29]。组蛋白去甲基化酶NO66使H3K4和H3K36去甲基化,调控包括Osx在内的成骨基因表达,抑制成骨分化[30]。 成骨相关信号分子也能诱导表观遗传修饰。Wnt5a诱导骨髓间充质干细胞中组蛋白甲基转移酶的磷酸化。SETDB1(KMT1E)利于组蛋白H3K9甲基化,抑制过氧化物酶体增殖物激活受体γ表达和脂源性分化,利于成骨分化[31]。 已经证实乙酰转移酶在成骨分化或骨形成中具有重要作用。CREB结合蛋白(CBP/KAT3A)是非特异性组蛋白乙酰转移酶,编码它的基因产生突变会导致Rubinstein-Taybi综合征,这是一种使脸部和肢体发育异常的常染色体显性遗传疾病[32]。CBP使组蛋白残基乙酰化,促进成骨过程中骨相关基因增强子的乙酰化和活化,是骨相关基因表达的直接或共调控因子[33]。还有几种乙酰转移酶在骨形成、成骨细胞相关基因表达或下调骨性关节炎等表观遗传调控方面发挥作用,包括PCAF(KAT2B)[34]、MOZ/MORF(KAT6A/KAT6B)和具有昼夜节奏规律的特异性乙酰转移酶Clock (KAT13D)[35]。 在骨科领域研究最多的组蛋白修饰酶是组蛋白去乙酰化酶。有研究已经明确特异性组蛋白去乙酰化酶能够调控骨相关基因、影响成骨细胞分化功能[36]。另外,组蛋白去乙酰化酶也能调控细胞外信号通路,在微环境中影响骨源性呈递和分化[37]。 正如许多乙酰转移酶一样,组蛋白去乙酰化酶功能具有多样性、复杂性,它可作用于非组蛋白上的乙酰化赖氨酸。许多组蛋白去乙酰化酶能使多种蛋白去乙酰化,包括骨形成相关转录因子Runx2,它能通过去除Runx2蛋白乙酰基或直接通过物理作用调控其功能[38]。因为大多数组蛋白去乙酰化酶蛋白本身不具有染色质结合能力,它们常作为染色质的转录共抑制因子出现。特异性组蛋白去乙酰化酶常与几种骨相关转录调控因子组成复合体,在微环境中调控成骨细胞相关基因表达,包括Runx2、NFATc1、Zfp521和Pbx1[39]。Runx2转录蛋白与DNA链直接结合,调控DNA链中碱性磷酸酶、Ⅰ型胶原、骨钙素等目的基因的成骨表达,在成骨发育中具有不可替代的作用。组蛋白去乙酰化酶6结合于Runx2蛋白的羧基端,构成组蛋白去乙酰化酶6/Runx2转录复合体,能够调控Runx2蛋白的促成骨活性(图1)。组蛋白去乙酰化酶不仅与成骨细胞成骨过程直接相关,还与骨性关节炎疾病有密切关系[40-41]。组蛋白去乙酰化酶亚型、与之结合的目标组蛋白及其在骨形成中的作用见表1。"
| [1] Kobayashi H, Kikyo N. Epigenetic regulation of open chromatin in pluripotent stem cells. Transl Res.2015;165(1): 18-27.[2] Mueller-Planitz F, Klinker H, Becker PB. Nucleosome sliding mechanisms: new twists in a looped history. Nat Struct Mol Biol. 2013;20(9):1026-1032.[3] Schaukowitch K, Kim TK. Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience. 2014;264:25-38.[4] Rothbart SB, Strahl BD. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014;1839(8): 627-643.[5] Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet.2010;11(3):204-220.[6] Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. 2013;341(6146):1237905.[7] Shirane K, Toh H, Kobayashi H, et al. Mouse oocyte methylomes at base resolution reveal genome-wide accumulation of non-CpG methylation and role of DNA methyltransferases. PLoS Genet. 2013 ;9(4):e1003439.[8] Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25(10):1010-1022.[9] Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet.2013;14(3):204-220.[10] Long HK, Blackledge NP, Klose RJ. ZF-CxxC domain-containing proteins, CpG islands and the chromatin connection. Biochem Soc Trans. 2013;41(3):727-740.[11] Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell .2014;156(1-2): 45-68.[12] Hashimoto K, Otero M, Imagawa K, et al. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1beta (IL1B) genes in chondrocytes depends onmethylation of specific proximal promoter CpG sites. J Biol Chem.2013;288(14):10061-10072.[13] Dansranjavin T, Krehl S, Mueller T, et al. The role of promoter CpG methylation in the epigenetic control of stem cell related genes during differentiation. Cell Cycle 2009;8(6):916-924.[14] Hsiao SH, Lee KD, Hsu CC, et al. DNA methylation of the Trip10 promoter accelerates mesenchymal stem cell lineage determination. Biochem Biophys Res Commun. 2010;400(3): 305-312.[15] Thaler R, Agsten M, Spitzer S, et al. Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and epigenetic DNA methylation. J Biol Chem. 2011;286(7):5578-5588.[16] Huang H, Sabari BR, Garcia BA, et al. SnapShot: histone modifications. Cell. 2014;159(2):458-458.e1.[17] Lee JS, Smith E, Shilatifard A. The language of histone crosstalk. Cell.2010;142(5):682-685.[18] Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet.2012;13(5): 343-357.[19] Schuettengruber B, Martinez AM, Iovino N, et al. Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol. 2011;12(12):799-814.[20] Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes Dev. 2013;27(12):1318-1338.[21] Lalonde ME, Cheng X, Cote J. Histone target selection within chromatin: an exemplary case of teamwork. Genes Dev. 2014;28(10):1029-1041.[22] Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381-395.[23] Choudhary C,Weinert BT, Nishida Y, et al. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol .2014;15(8):536-550.[24] Meyer MB, Benkusky NA, Pike JW. The RUNX2 cistrome in osteoblasts: characterization, down-regulation following differentiation, and relationship to gene expression. J Biol Chem. 2014;289(23):16016-16031.[25] Ito T, Yadav N, Lee J, et al. Arginine methyltransferase CARM1/PRMT4 regulates endochondral ossification. BMC Dev Biol. 2009;9:47.[26] Nimura K, Ura K, Shiratori H, et al. A histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf–Hirschhorn syndrome. Nature.2009;460(7252):287-291.[27] Wei Y, Chen YH, Li LY, et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13(1):87-94.[28] Hemming S, Cakouros D, Isenmann S, et al.EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells .2014;32(3): 802-815.[29] Yang D, Okamura H, Nakashima Y, et al. Histone demethylase Jmjd3 regulates osteoblast differentiation via transcription factors Runx2 and osterix. J Biol Chem.2013; 288(47):33530-33541.[30] Sinha KM, Yasuda H, Zhou X, et al. Osterix and NO66 histone demethylase control the chromatin of Osterix target genes during osteoblast differentiation. J Bone Miner Res .2014; 29(4): 855-865.[31] Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol . 2007;9(11):1273-1285.[32] Park E, Kim Y, Ryu H, et al. Epigenetic mechanisms of Rubinstein-Taybi syndrome. Neuromolecular Med. 2014;16(1): 16-24.[33] Rodriguez-Carballo E, Ulsamer A, Susperregui AR, et al. Conserved regulatory motifs in osteogenic gene promoters integrate cooperative effects of canonical Wnt and BMP pathways. J Bone Miner Res.2011;26(4):718-729.[34] Wang CY, Yang SF, Wang Z, et al. PCAF acetylates Runx2 and promotes osteoblast differentiation. J Bone Miner Metab. 2013;31(4):381-389.[35] Campeau PM, Kim JC, Lu JT, et al. Mutations in KAT6B, encoding a histone acetyltransferase, cause Genitopatellar syndrome. Am J Hum Genet. 2012;90(2):282-289[36] Bradley EW, Carpio LR, Olson EN, et al. Histone deacetylase 7 (Hdac7) suppresses chondrocyte proliferation and β-catenin activity during endochondral ossification. J Biol Chem.2015; 290(1):118-126.[37] Dudakovic A, Camilleri ET, Lewallen EA, et al. Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. J Cell Physiol .2015;230(1):52-62.[38] Pham L, Kaiser B, Romsa A, et al. HDAC3 and HDAC7 have opposite effects on osteoclast differentiation. J Biol Chem. 2011; 286(14):12056-12065.[39] Hesse E, Saito H, Kiviranta R, et al. Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity. J Cell Biol .2010;191(7): 1271-1283.[40] Chen WP, Bao JP, Hu PF, et al. Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis. Mol Biol Rep. 2010;37(8):3967-3972.[41] Culley KL, Hui W, Barter MJ, et al. Class I histone deacetylase inhibition modulates metalloproteinase expression and blocks cytokine-induced cartilage degradation. Arthritis Rheum. 2013;65(7):1822-1830.[42] Zhang YX, Sun HL, Liang H, et al. Dynamic and distinct histone modifications of osteogenic genes during osteogenic differentiation. J Biochem. 2015;158(6):445-457. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Cao Wei, Mao Furong, Hu Xiaohua, Yang Xiaohong. N-6 methyladenosine RNA methylation regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 266-270. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||