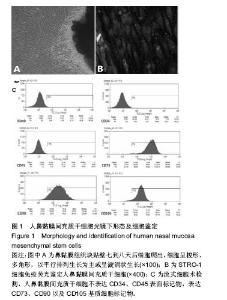
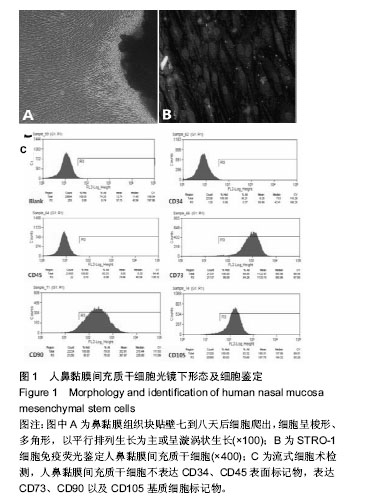
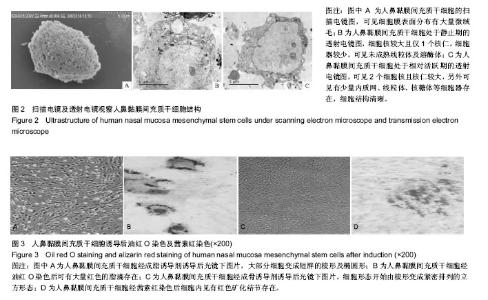
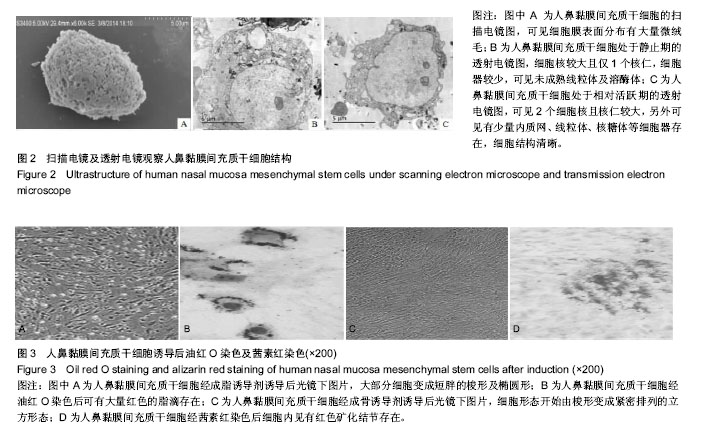
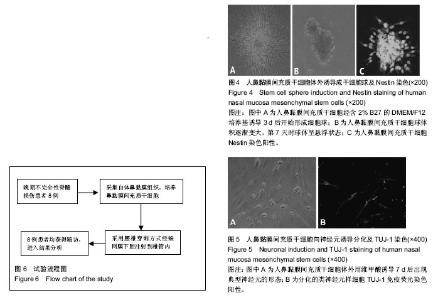
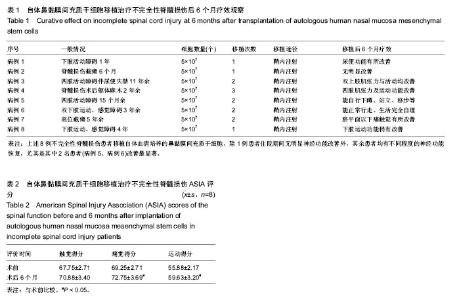
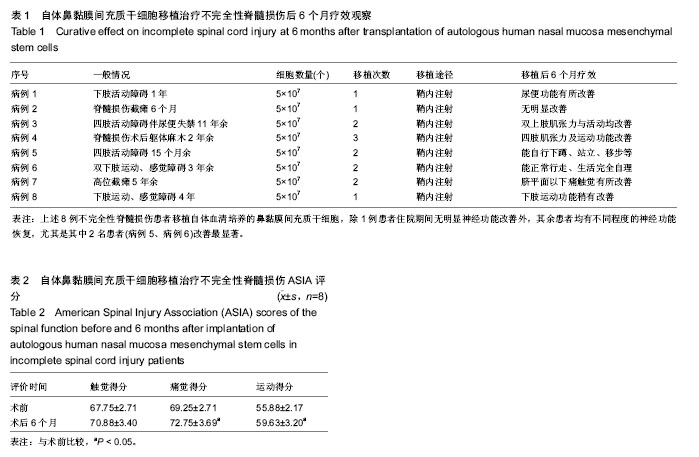
| [1] Veerakumar A, Cheng JJ, Sunshine A, et al. Baclofen dosage after traumatic spinal cord injury: a multi-decade retrospective analysis. Clin Neurol Neurosurg. 2015;129:50-56.[2] Hou S, Tom VJ, Graham L, et al. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci. 2013; 33(43):17138-17149.[3] Fayyad-Kazan H, Faour WH, Badran B, et al. The immunomodulatory properties of human bone marrow-derived mesenchymal stromal cells are defined according to multiple immunobiological criteria. Inflamm Res. 2016;65(6):501-510.[4] Ge L, Jiang M, Duan D, et al. Secretome of Olfactory Mucosa Mesenchymal Stem Cell, a Multiple Potential Stem Cell. Stem Cells Int. 2016;2016:1243659.[5] Lee PH, Kim JW, Bang OY, et al. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83(5):723-730.[6] Penha EM, Meira CS, Guimarães ET, et al. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014;2014:437521.[7] 罗树伟,谢常青,卢光琇.人胚神经干细胞的分离培养和鉴定[J]. 中南大学学报:医学版, 2004,29(2):129-131.[8] 葛丽特,卓毅,段答,等.人嗅黏膜间充质干细胞的生物学特性[J].中南大学学报:医学版, 2015,40(1): 53-58.[9] Bas E, Van De Water TR, Lumbreras V, et al. Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion. Stem Cells Dev. 2014;23(5):502-514.[10] 何旭,杨旭芳,张丽红,等.人脂肪干细胞的形态学特征[J].生物医学工程学杂志,2011,28(2): 337-341.[11] Zeng HL, Zhong Q, Qin YL, et al. Hypoxia-mimetic agents inhibit proliferation and alter the morphology of human umbilical cord-derived mesenchymal stem cells. BMC Cell Biol. 2011;12:32.[12] Shafiee A, Kabiri M, Ahmadbeigi N, et al. Nasal septum-derived multipotent progenitors: a potent source for stem cell-based regenerative medicine. Stem Cells Dev. 2011;20(12):2077-2091.[13] Donega V, Nijboer CH, van Tilborg G, et al. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol. 2014;261:53-64.[14] Zemel'ko VI, Kozhukharova IV, Kovaleva ZV, et al. BDNF secretion in human mesenchymal stem cells isolated from bone marrow, endometrium and adipose tissue. Tsitologiia. 2014;56(3):204-211.[15] Himes BT, Neuhuber B, Coleman C, et al. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20(2):278-296.[16] Tavakoli F, Ostad SN, Khori V, et al. Outcome improvement of cellular cardiomyoplasty using triple therapy: mesenchymal stem cell+erythropoietin+vascular endothelial growth factor. Eur J Pharmacol. 2013;714(1-3):456-463.[17] Saito F, Nakatani T, Iwase M, et al. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neurosci. 2012;30(2):127-136.[18] Saito F, Nakatani T, Iwase M, et al. Spinal cord injury treatment with intrathecal autologous bone marrow stromal cell transplantation: the first clinical trial case report. J Trauma. 2008;64(1):53-59.[19] Pal R, Gopinath C, Rao NM, et al. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy. 2010;12(6):792-806.[20] Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells. Osteoarthritis Cartilage. 2005;13(10):845-853.[21] Zhang W, Zeng YS, Zhang XB, et al. Combination of adenoviral vector-mediated neurotrophin-3 gene transfer and retinoic acid promotes adult bone marrow cells to differentiate into neuronal phenotypes. Neurosci Lett. 2006;408(2):98-103.[22] Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87-117. |