Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (16): 2509-2514.doi: 10.3969/j.issn.2095-4344.2017.16.009
Previous Articles Next Articles
Influence of different intensities of extracorporeal shock waves on the osteogenesis ability of alveolar osteoblasts
Guo Yuan1, Zhang Chi2, Liu Song2, Wang Zhao2, Pan Xing-fei3, Wang Le2
- 1Department of Stomatology, 2Department of Orthopedics, 3Department of Infectious Diseases, the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China
-
Revised:2017-03-10Online:2017-06-08Published:2017-07-06 -
Contact:Wang Le, M.D., Associate chief physician, Department of Orthopedics, the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China -
About author:Guo Yuan, Master, Attending physician, Department of Stomatology, the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 31570980; the Public Research and Construction Project of Guangdong Province, No. 2014A020212347; the Scientific Research Project of Universities in Guangzhou, No. 1201430092
CLC Number:
Cite this article
Guo Yuan, Zhang Chi, Liu Song, Wang Zhao, Pan Xing-fei, Wang Le. Influence of different intensities of extracorporeal shock waves on the osteogenesis ability of alveolar osteoblasts[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(16): 2509-2514.
share this article
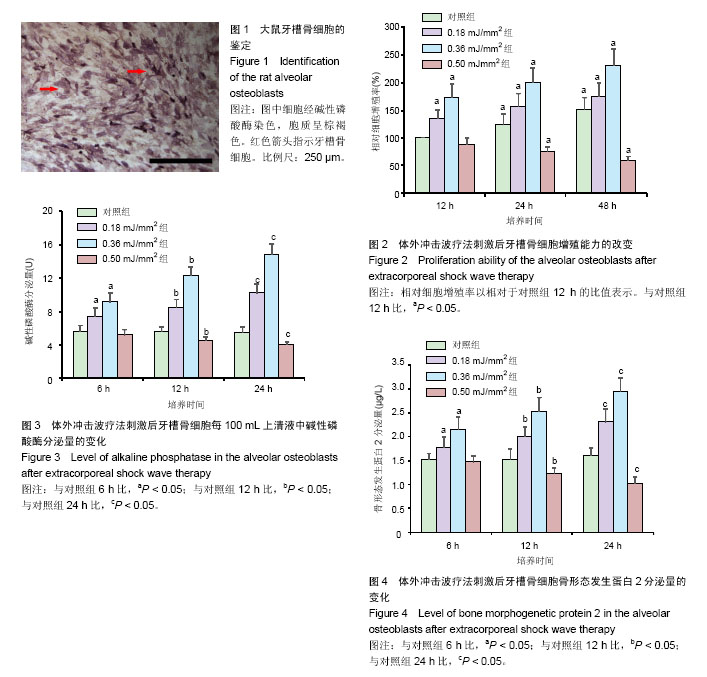
2.1 牙槽骨细胞碱性磷酸酶染色定性 第3代大鼠牙槽骨细胞呈梭形贴壁生长,碱性磷酸酶染色阳性(图1)。 2.2 体外冲击波疗法刺激后牙槽骨细胞增殖能力改变 大鼠牙槽骨细胞经不同强度的体外冲击波疗法刺激后表现出不同的增殖能力变化。当采用强度为0.18 mJ/mm2和0.36 mJ/mm2的体外冲击波疗法作用于细胞时,随着作用强度上升,在观察时间点内,大鼠牙槽骨细胞增殖能力明显上升(P < 0.05)。当体外冲击波疗法强度达到 0.50 mJ/mm2时,随着培养时间的延长,细胞增殖能力出现明显下降(P < 0.05;图2)。 2.3 体外冲击波疗法刺激后牙槽骨细胞碱性磷酸酶分泌量的变化 与对照组相比,强度为0.18 mJ/mm2和0.36 mJ/mm2的体外冲击波疗法作用于细胞后,可明显促进牙槽骨细胞分泌碱性磷酸酶(P < 0.05)。当体外冲击波疗法强度达到0.50 mJ/mm2时,细胞分泌碱性磷酸酶的量明显下降(P < 0.05;图3)。 2.4 体外冲击波疗法刺激后牙槽骨细胞骨形态发生蛋白2分泌量的变化 ELISA结果显示,与对照组相比,强度为0.18 mJ/mm2和0.36 mJ/mm2的体外冲击波疗法作用于细胞后,可明显促进牙槽骨细胞分泌骨形态发生蛋白2(P < 0.05)。当体外冲击波疗法强度达到0.50 mJ/mm2时,细胞分泌骨形态发生蛋白2量明显下降(P < 0.05;图4)。"
| [1] Türk C,Pet?ík A,Sarica K,et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69(3):475-482. [2] Yu C,Longfei L,Long W,et al. A systematic review and meta-analysis of new onset hypertension after extracorporeal shock wave lithotripsy. Int Urol Nephrol. 2014;46(4):719-725. [3] D Agostino MC,Frairia R,Romeo P,et al. Extracorporeal shockwaves as regenerative therapy in orthopedic traumatology: a narrative review from basic research to clinical practice. J Biol Regul Homeost Agents. 2016;30(2):323-332. [4] Alkhawashki HM. Shock wave therapy of fracture nonunion. Injury. 2015;46(11):2248-2252. [5] Bach C,Karaolides T,Buchholz N. Extracorporeal shock wave lithotripsy: What is new? Arab J Urol. 2012;10(3):289-295. [6] Zhai L,Ma XL,Jiang C,et al. Human autologous mesenchymal stem cells with extracorporeal shock wave therapy for nonunion of long bones. Indian J Orthop. 2016;50(5):543-550.[7] Barnes K,Lanz O,Werre S,et al. Comparison of autogenous cancellous bone grafting and extracorporeal shock wave therapy on osteotomy healing in the tibial tuberosity advancement procedure in dogs. Radiographic densitometric evaluation. Vet Comp Orthop Traumatol. 2015;28(3):207-214. [8] 李光辉,潘晓岗.参与正畸牙移动的相关因子研究进展[J].口腔医学,2015,35(6):508-512.[9] 刘奉,蒋祥林,刘明明.不同培养方法对人脐带间充质干细胞的影响[J].中国组织工程研究,2016,20(28):4136-4141.[10] Simon MJ, Beil FT, Riedel C, et al. Deterioration of teeth and alveolar bone loss due to chronic environmental high-level fluoride and low calcium exposure. Clin Oral Investig. 2016; 20(9):2361-2370.[11] Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85(7):596-607.[12] Jahanbin A,Rashed R,Alamdari DH,et al. Success of Maxillary Alveolar Defect Repair in Rats Using Osteoblast-Differentiated Human Deciduous Dental Pulp Stem Cells. J Oral Maxillofac Surg. 2016;74(4):829.e1-9. [13] Li X,Chen M,Li L,et al. Extracorporeal shock wave therapy: a potential adjuvant treatment for peri-implantitis. Med Hypotheses. 2010;74(1):120-122. [14] 刘长剑,刘建国.体外冲击波原理与成骨[J].中国骨与关节损伤杂志,2007,22(9):788-790.[15] 王五洲,邢更彦.体外冲击波与成骨活性因子[J].中华物理医学与康复杂志,2007,20(10):784-786.[16] Blumhardt S,Frey DP,Toniolo M,et al. Safety and efficacy of extracorporeal shock wave therapy (ESWT) in calcinosis cutis associated with systemic sclerosis. Clin Exp Rheumatol. 2016; 34 Suppl 100(5):177-180. [17] Chen YL,Chen KH,Yin TC,et al. Extracorporeal shock wave therapy effectively prevented diabetic neuropathy. Am J Transl Res. 2015;7(12):2543-2560.[18] Ikeda K,Tomita K,Takayama K. Application of extracorporeal shock wave on bone: preliminary report. J Trauma. 1999; 47(5):946-950.[19] Chen Y,Xu J,Huang Z,et al. An Innovative Approach for Enhancing Bone Defect Healing Using PLGA Scaffolds Seeded with Extracorporeal-shock-wave-treated Bone Marrow Mesenchymal Stem Cells (BMSCs). Sci Rep. 2017;7:44130. [20] Huang HM,Li XL,Tu SQ,et al. Effects of Roughly Focused Extracorporeal Shock Waves Therapy on the Expressions of Bone Morphogenetic Protein-2 and Osteoprotegerin in Osteoporotic Fracture in Rats. Chin Med J (Engl). 2016; 129(21):2567-2575.[21] Sansone V,Romeo P,Lavanga V. Extracorporeal Shock Wave Therapy Is Effective in the Treatment of Bone Marrow Edema of the Medial Compartment of the Knee: A Comparative Study. Med Princ Pract. 2017;26(1):23-29.[22] Sun W,Gao F,Guo W,et al. Focused extracorporeal shock wave for osteonecrosis of the femoral head with leukemia after allo-HSCT: a case series. Bone Marrow Transplant. 2016;51(11):1507-1509.[23] Ma HZ,Zhou DS,Li D,et al. A histomorphometric study of necrotic femoral head in rabbits treated with extracorporeal shock waves. J Phys Ther Sci. 2017;29(1):24-28.[24] Tamma R,dell'Endice S,Notarnicola A,et al. Extracorporeal shock waves stimulate osteoblast activities. Ultrasound Med Biol. 2009;35(12):2093-2100. [25] 宋择众.通过检测碱性磷酸酶和骨钙素2的表达水平定量分析组织工程骨成骨影响因素[D].济南:山东大学,2015.[26] 张晓,刘云松,吕珑薇,等.骨形态发生蛋白2/7异二聚体对人脂肪间充质干细胞成骨分化的促进作用[J].北京大学学报医学版, 2016,48(1):37-44.[27] Altunta? EE,Oztemur Z,Ozer H,et al. Effect of extracorporeal shock waves on subcondylar mandibular fractures. J Craniofac Surg. 2012;23(6):1645-1648. [28] Lai JP,Wang FS,Hung CM,et al. Extracorporeal shock wave accelerates consolidation in distraction osteogenesis of the rat mandible. J Trauma. 2010;69(5):1252-1258. [29] Hu TX,Li ZB,Li Z. Time effect of dentin matrix protein 1 and osteoclast expression during mandibular fracture healing in rats. Zhonghua Kou Qiang Yi Xue Za Zhi. 2007;42(10): 631-632. [30] Rowshan HH,Parham MA,Baur DA,et al. Effect of intermittent systemic administration of recombinant parathyroid hormone (1-34) on mandibular fracture healing in rats. J Oral Maxillofac Surg. 2010;68(2):260-267. [31] Okta? B,Orhan Z,Erbil B,et al. Effect of extracorporeal shock wave therapy on fracture healing in rat femural fractures with intact and excised periosteum. Eklem Hastalik Cerrahisi. 2014;25(3):158-162. [32] Reynders P, Becker JH, Broos P. Osteogenic ability of free periosteal autografts in tibial fractures with severe soft tissue damage: an experimental study. J Orthop Trauma. 1999; 13(2):121-128. [33] Neagu TP, Enache V, Cocolo? I, et al. Experimental study in order to assess the effects of limited periosteum stripping on the fracture healing and to compare osteosynthesis using plates and screws with intramedullary Kirschner wire fixation. Rom J Morphol Embryol. 2016;57(2):437-443. [34] Beutler S, Regel G, Pape HC, et al. Extracorporeal shock wave therapy for delayed union of long bone fractures - preliminary results of a prospective cohort study. Unfallchirurg. 1999;102(11):839-847. [35] Klein M, Stieger A, Stenger D, et al. Comparison of healing process in open osteotomy model and open fracture model: Delayed healing of osteotomies after intramedullary screw fixation. J Orthop Res. 2015;33(7):971-978. [36] Kuo SJ, Su IC, Wang CJ, et al. Extracorporeal shockwave therapy (ESWT) in the treatment of atrophic non-unions of femoral shaft fractures. Int J Surg. 2015;24(Pt B):131-134. [37] Reynders P, Becker J, Broos P. The osteogenic potential of free periosteal autografts in tibial fractures with severe soft tissue damage: an experimental study. Acta Orthop Belg. 1998;64(2):184-192. [38] Kamiya N, Takagi M. Differential expression of dentin matrix protein 1, type I collagen and osteocalcin genes in rat developing mandibular bone. Histochem J. 2001;33(9-10): 545-552. [39] Li S, Liu S, Tian W, et al. A study on mRNA expression levels of integrin beta 1 during the healing process of mandibular fractures. Hua Xi Kou Qiang Yi Xue Za Zhi. 2001;19(6): 351-353.[40] Liu S, Li S, Cheng G. Immunohistochemical research of integrin alpha 5 expression during mandibular fracture healing. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21(2):101-103.[41] Zheng L, Yamashiro T, Fukunaga T, et al. Bone morphogenetic protein 3 expression pattern in rat condylar cartilage, femoral cartilage and mandibular fracture callus. Eur J Oral Sci. 2005;113(4):318-325.[42] Heybeli N, Ye?ilda? A, Oyar O, et al. Diagnostic ultrasound treatment increases the bone fracture-healing rate in an internally fixed rat femoral osteotomy model. J Ultrasound Med. 2002;21(12):1357-1363. [43] Nakazawa T, Nakajima A, Shiomi K, et al. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37(5):711-719. [44] Yun JI, Wikesjö UM, Borke JL, et al. Effect of systemic parathyroid hormone (1-34) and a beta-tricalcium phosphate biomaterial on local bone formation in a critical-size rat calvarial defect model. J Clin Periodontol. 2010;37(5): 419-426. [45] Komrakova M, Stuermer EK, Werner C, et al. Effect of human parathyroid hormone hPTH (1-34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone. 2010;47(3):480-492. [46] Hashimoto T, Shigetomi M, Ohno T, et al. Sequential treatment with intermittent low-dose human parathyroid hormone (1-34) and bisphosphonate enhances large-size skeletal reconstruction by vascularized bone transplantation. Calcif Tissue Int. 2007;81(3):232-239. [47] Lin EA, Liu CJ, Monroy A, et al. Prevention of atrophic nonunion by the systemic administration of parathyroid hormone (PTH 1-34) in an experimental animal model. J Orthop Trauma. 2012;26(12):719-723. [48] Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14(6):960-968. [49] Li N, Wang MY, He L, et al. Intermittent low-dose administration of recombinant human parathyroid hormone (1-34) promotes the expression of Runx2 during early stage of fracture healing. Zhonghua Yi Xue Za Zhi. 2009;89(11): 771-776. [50] Tägil M, McDonald MM, Morse A, et al. Intermittent PTH(1-34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone. 2010;46(3):852-859. [51] Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am. 2005;87(4):731-741. [52] Islam AA, Rasubala L, Yoshikawa H, et al. Healing of fractures in osteoporotic rat mandible shown by the expression of bone morphogenetic protein-2 and tumour necrosis factor-alpha. Br J Oral Maxillofac Surg. 2005;43(5): 383-391. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [5] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [6] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [7] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [8] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [9] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [10] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [11] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [12] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Feng Dongfei, He Hongxu, Xie Qi, Zhang Lili, Zhou Hui, Li Wei. Selection of key genes related to biological functions and regulation pathway in periodontal reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 253-259. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||