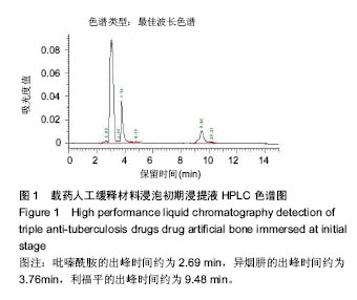
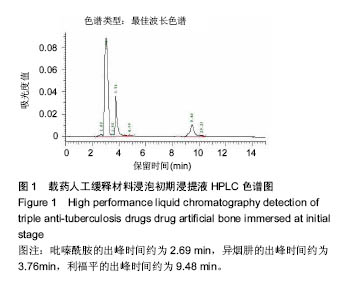
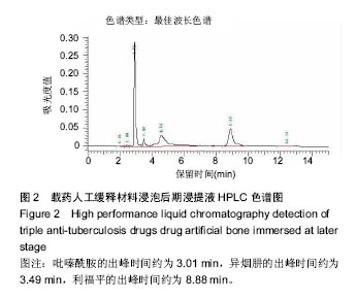
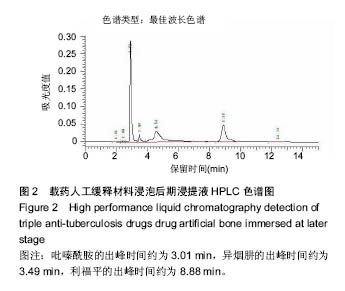
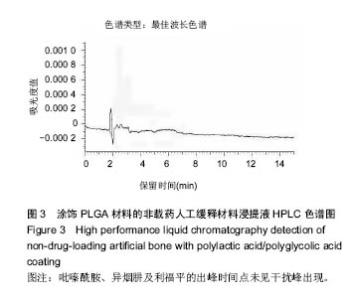
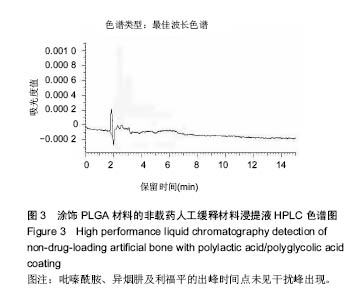
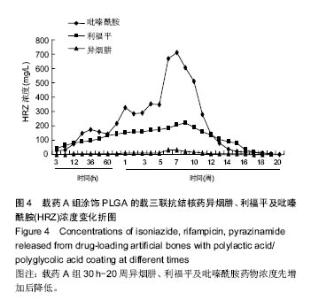
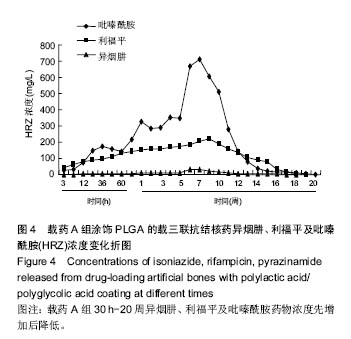
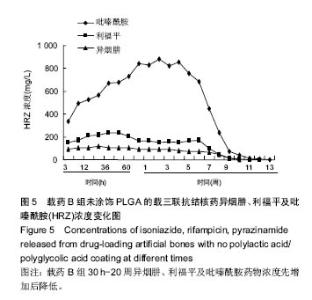
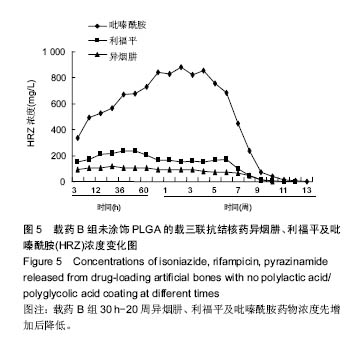
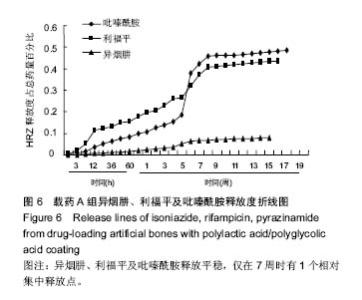
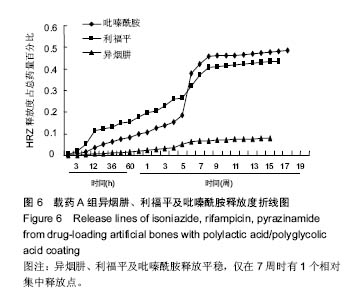
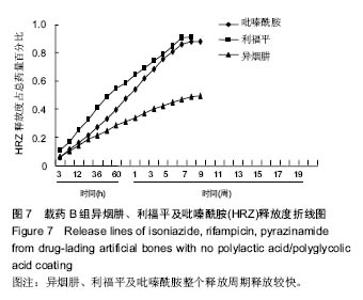
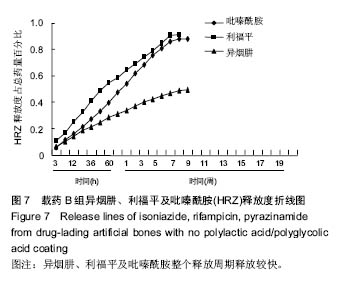
| [1]Diacon AH, Donald PR. The early bactericidal activity of antituberculosis drugs. Exp Rev Anti Infect Ther. 2014;12(2): 223-237.[2]Ge Z, Wang Z, Wei M. Measurement of the concentration of three antituberculosis drugs in the focus of spinal tuberculosis. Eur Spine J. 2008;17(11):1482-1487.[3]龙娜,吕竹芬.微球缓释系统的突释现象及其影响因素[J].中国药师,2010,13(3):421-423.[4]德向研,施建党,王自立,等.载三联抗结核药硫酸钙/氨基酸聚合物人工骨体内缓释实验研究[J].中国脊柱脊髓杂志,2013,23(6): 531-536.[5]刘海涛,王自立,施建党,等.人工骨载三联抗结核药物含量对其抗机械压缩强度的影响[J].宁夏医科大学学报, 2013,35(7): 759-762.[6]刘海涛,施建党,王骞,等.载三联抗结核药物硫酸钙/聚氨基酸人工材料体外缓释性能的观察[J].中国脊柱脊髓杂志, 2015,25(3): 239-244.[7]Chen J, Gao J, Yin H, et al. Size-controlled preparation of α-calcium sulphate hemihydrate starting from calcium sulphate dihydrate in the presence of modifiers and the dissolution rate in simulated body fluid. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3256-3262.[8]Hirota K, Hasegawa T, Nakajima T, et al. Delivery of rifampicin-PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J Control Release. 2010;142(3):339-346.[9]赵锡江,齐新生,董寅生.rhBMP-2/PLGA缓释支架的制备及对MSCs细胞成骨活性的体外研究[J].国际生物医学工程杂志, 2010,33(3):147-151.[10]Ratier A, Freche M, Lacout JL, et al. Behaviour of an injectable calcium phosphate cement with added tetracycline. Int J Pharm. 2004;274(1-2):261-268.[11]Joosten U, Joist A, Frebel T, et al. Evaluation of an in situ setting injectable calcium phosphate as a new carrier materials for gentamicin in the treatment of chronic osteomyelitis: studies in vitro and in vivo. Biomaterials. 2004; 25(18):4287-4295.[12]Krasko MY, Golenser J, Nyska A, et al. Gentamicin extended release from an injectable polymericim plant. J Control Release. 2007;117(1):90-96.[13]曹丰亮,席延伟,唐琳,等.姜黄素肺靶向明胶微球的制备及性质研究[J].中药材,2009,3(32):423-426.[14]黄莹莹,齐民,杨大智,等.冠脉支架表面PLGA涂层制备及其血液相容性研究[J].功能材料,2006,37(3):411-414.[15]张峻山,王自立,施建党,等.复合三联抗结核药人工缓释材料体外抗结核性能[J].脊柱外科杂志,2014,12(6):380-384.[16]Geng G, Wang Q, Shi J, et al. Establishment of a new zealand rabbit model of spinal tuberculosis. J Spinal Disord Tech. 2015; 28(3):E140-E145.[17]刘海涛,施建党,王骞,等.硫酸钙/聚氨基酸复合三联抗结核药人工缓释材料的制备及物理性能测定[J].中国矫形外科杂志,2015, 23(21):1984-1988.[18]张立群,李华,巩维进.结核病的化学治疗[J].中国临床医生,2009, 37(21):165-166.[19]周林.抗结核药物研究进展[J].中国临床医生,2013,41(3):14-17.[20]李群力,蒋晓萌,施存元.高效液相色谱法测定复方利福平片中利福平、异烟肼及吡嗪酰胺的含量[J].中国抗生素杂志,2002,27(9): 570-571.[21]Guo H, Wei J, Liu CS. Development of a degradable cement of calcium phosphate and calcium sulfate composite for bone reconstruction. Biomed Mater. 2006;1(4):193-197.[22]Urban RM, Turner TM, Hall DJ, et al. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin Orthop Relat Res. 2008;459:110-117.[23]Civinini R, De B, Carulli C, et al. The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head. Int Orthop. 2012;36(8):1583-1588.[24]张卓,孙宇航,耿广起,等.载三联抗痨药硫酸钙/聚氨基酸人工骨植入脊柱结核病灶内:植骨界面的组织学改变[J].中国组织工程研究,2016,20(47):7027-7033.[25]应吕方,贾叙锋,程尹,等.硫酸钙人工骨复合培养骨髓间充质干细胞对骨诱导脊柱融合的影响[J].中国组织工程研究,2016,20(43): 6396-6402. |