Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (2): 232-237.doi: 10.3969/j.issn.2095-4344.2017.02.013
Previous Articles Next Articles
Chitin hybrid membrane carrying cells repairs corneal epithelial injury
- 1Department of Ophthalmology, 2Department of Orthopaedics, the Affiliated Hospital of Hebei University, Baoding 071000, Hebei Province, China
-
Received:2016-10-25Online:2017-01-18Published:2017-02-27 -
Contact:Wu Shi-ke, Attending physician, Department of Orthopaedics, the Affiliated Hospital of Hebei University, Baoding 071000, Hebei Province, China -
About author:Lu Jing, Master, Attending physician, Department of Ophthalmology, the Affiliated Hospital of Hebei University, Baoding 071000, Hebei Province, China -
Supported by:the Key Medical Research Project of Hebei Province, No. zl20140018
CLC Number:
Cite this article
Lu Jing, Wu Shi-ke, Chen Guang, Zhao Yue, Li Dan.
share this article
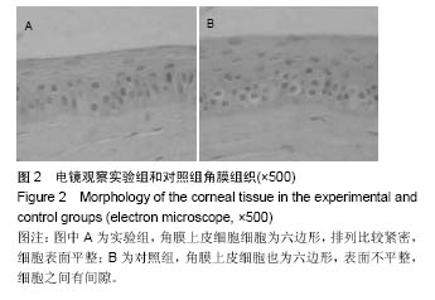
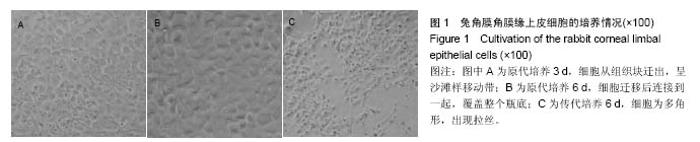
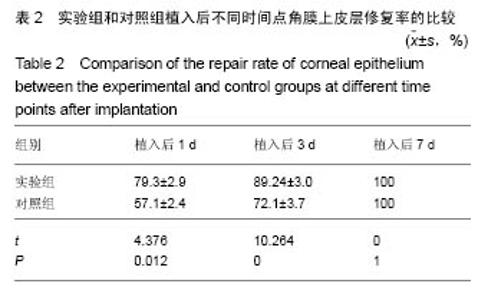
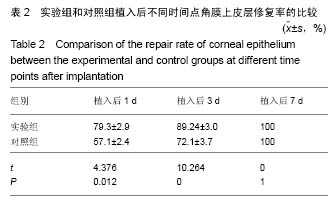
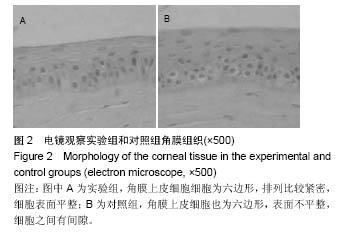
2.2 体内修复实验结果 2.2.1 实验动物数量分析 18只兔均造模成功,并进入结果分析。 2.2.2 两组眼大体观察结果 植入后1 d,实验组眼血管充血,轻度畏光,分泌物不多,没有水肿;对照组眼血管充血明显,畏光,分泌物比较多,角膜浑浊,水肿现象明显。植入后3 d,实验组眼充血明显减轻,没有畏光,分泌物减少,没有水肿;对照组眼仍明显充血,畏光,分泌物仍然比较多,仍有角膜浑浊和水肿。植入后7 d,两组眼看起来都恢复正常。 2.2.3 两组眼超微结构观察结果 植入后7 d,实验组角膜上皮细胞细胞为六边形,排列比较紧密,细胞表面平整;对照组角膜上皮细胞也为六边形,表面不平整,细胞之间有间隙,见图2。"
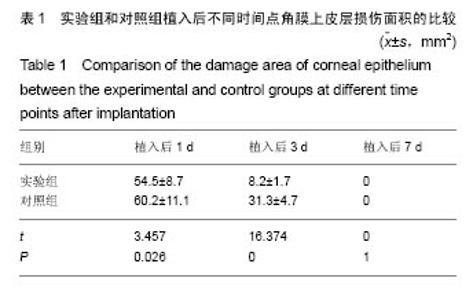
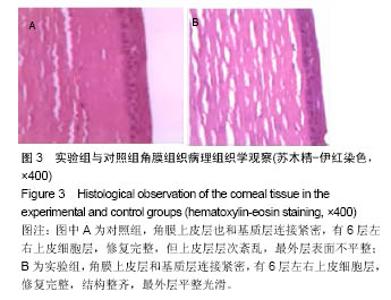
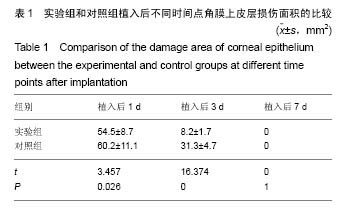
2.2.5 两组角膜损伤区荧光染色结果 植入后1 d时,实验组角膜上皮层边缘开始出现比较明显的修复现象;对照组角膜上皮层的损伤面积基本没有变化,没有修复现象出现。植入后2 d时,实验组角膜上皮层大部分已被修复,损伤上皮层的修复是分散的,与对照组的从角膜缘细胞移行修复明显不同,修复面积明显大于对照组;对照组角膜上皮层损伤面积开始出现修复现象,修复从角膜缘细胞开始向损伤中心移行,表明实验组移植的上皮细胞可能在损伤角膜上皮层修复中发挥了一定作用。植入后3 d时,实验组角膜上皮层基本恢复正常状态;对照组角膜上皮层的损伤面积也明显减少,但修复速度明显较实验组慢。植入后第4天,实验组角膜上皮层已完全恢复正常状态;对照组角膜上皮层损伤大部分被修复,只有少量损伤区域。植入后第五六天,对照组角膜上皮层已基本修复正常,植入后第7天,完全修复正常。表明甲壳素载细胞膜参与了大白兔损伤角膜上皮层的修复,能够加快角膜损伤上皮层的修复速度,缩短修复时间。 2.2.6 两组角膜上皮层损伤面积与修复率的比较 角膜上皮层损伤面积:实验组植入后1 ,3 d的角膜上皮层损伤面积明显小于对照组(t=3.457,16.374,P均< 0.05),见表1。"
| [1]Torricelli AA,Santhanam A,Wu J,et al.The corneal fibrosis response toepithelial-stromal injury.Exp Eye Res. 2016;142: 110-118.[2]孙亚杰,李爱朋,潘志强.几种抗炎滴眼液对兔角膜上皮损伤和愈合影响的实验研究[J].眼科,2012,21(3):166-171.[3]穆剑,王梅,胡玉新,等.角膜上皮损伤引起复发性角膜上皮糜烂12例分析[J].广州医药,2012,43(4):29-30.[4]Xiang J,Le Q,Li Y,et al.In vivo confocal microscopy of early cornealepithelial recovery in patients with chemical injury.Eye (Lond).2015;29(12):1570-1578. [5]Hua X,Deng R,Li J,et al.Protective Effects of L-Carnitine Against OxidativeInjury by Hyperosmolarity in Human CornealEpithelial Cells.Invest Ophthalmol Vis Sci. 2015;56(9): 5503-5511. [6]Zhu KY,Merzendorfer H,Zhang W,et al.Biosynthesis, Turnover, and Functions of Chitin in Insects.Annu Rev Entomol.2016;61: 177-196. [7]Zhang W,Wang Y,Sui X,et al.Effects of chitin and sepia ink hybrid sponge on the healing of burning wound rats and its impact on macrophages in vitro.Acta Cir Bras. 2016;31(2): 119-125. [8]Sayari N,Sila A,Abdelmalek BE,et al.Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities.Int J Biol Macromol. 2016;87:163-171. [9]Usman A,Zia KM,Zuber M,et al.Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications.Int J Biol Macromol.2016;86: 630-645. [10]隋鲜鲜.甲壳素载体支架的生物相容性和对角膜上皮损伤的修复研究[D].中国海洋大学, 2013.[11]吴伟,李朝阳,王智崇,等.小牛血去蛋白提取物与重组人表皮生长因子促进兔角膜上皮损伤愈合和血管新生[J].眼科新进展,2014, 34(5):401-404. [12]Oh JY,Ko JH,Kim MK,et al.Effects of mesenchymal stem/stromal cells on cultures of corneal epithelial progenitor cells with ethanol injury.Invest Ophthalmol Vis Sci. 2014; 55(11): 7628-7635. [13]Sumioka T,Okada Y,Reinach PS,et al.Impairment of corneal epithelialwound healing in a TRPV1-deficient mouse.Invest Ophthalmol Vis Sci. 2014;55(5):3295-3302.[14]黄晨晨,廖荣丰,汪枫,等.糖尿病大鼠角膜上皮损伤修复过程中occludin的重塑特点[J].安徽医科大学学报, 2015,50(11): 1583-1587.[15]Song IS,Kang SS,Kim ES,et al.Heat shock protein 27 phosphorylation is involved in epithelial cell apoptosis as well as epithelialmigration during cornealepithelial wound healing. Exp Eye Res.2014;118:36-41. [16]Kim TH,Park YW,Ahn JS,et al.Effects of conditioned media from human amnioticepithelial cells on corneal alkali injuries in rabbits.J Vet Sci.2013;14(1):61-67.[17]Gehlsen U,Oetke A,Szaszák M,et al.Two-photon fluorescence lifetime imaging monitors metabolic changes during wound healing ofcorneal epithelial cells in vitro.Graefes Arch Clin Exp Ophthalmol. 2012;250(9): 1293-1302. [18]Zhang Y,Kobayashi T,Hayashi Y,et al.Important role of epiregulin in inflammatory responses during corneal epithelial wound healing.Invest Ophthalmol Vis Sci. 2012;53(4): 2414-2423. [19]Kadar T,Horwitz V,Sahar R,et al.Delayed loss of corneal epithelialstem cells in a chemical injury model associated with limbal stem celldeficiency in rabbits.Curr Eye Res.2011; 36(12):1098-1107. [20]Szabó DJ,Noer A,Nagymihály R,et al.Long-Term Cultures of Human CorneaLimbal Explants Form 3D Structures Ex Vivo - Implications for Tissue Engineering and Clinical Applications.PLoS One. 2015;10(11):e0143053. [21]Miotto M,Gouveia RM,Connon CJ.Peptide Amphiphiles in Corneal Tissue Engineering.J Funct Biomater.2015;6(3): 687-707. [22]Levis HJ,Kureshi AK,Massie I,et al.Tissue Engineering the Cornea: The Evolution of RAFT.J Funct Biomater.2015; 6(1):50-65. [23]Parke-Houben R,Fox CH,Zheng LL,et al. Interpenetrating polymer network hydrogel scaffolds for artificial cornea periphery.J Mater Sci Mater Med. 2015;26(2):107. [24]Li W,Long Y,Liu Y,et al.Fabrication and characterization of chitosan-collagen crosslinked membranes for corneal tissue engineering.J Biomater Sci Polym Ed. 2014;25(17): 1962-1972. [25]颜静.明胶/PLLA复合纤维膜的制备及在角膜组织工程中的性能研究[D].吉林大学,2012.[26]张孟雨.组织工程角膜支架材料研究进展[J].现代妇女(下旬), 2015, 31(1):230.[27]Long K,Liu Y,Li W,et al.Improving the mechanical properties of collagen-based membranes using silk fibroin for cornealtissue engineering.J Biomed Mater Res A. 2015; 103(3):1159-1168. [28]Salehi S,Grünert AK,Bahners T,et al.New nanofibrous scaffold for cornealtissue engineering.Klin Monbl Augenheilkd. 2014;231(6):626-630. [29]Wang HY,Wei RH,Zhao SZ.Evaluation of corneal cell growth on tissue engineering materials as artificial cornea scaffolds. Int J Ophthalmol.2013;6(6):873-878. [30]Tonsomboon K,Strange DG,Oyen ML.Gelatin nanofiber-reinforced alginate gel scaffolds for corneal tissue engineering.Conf Proc IEEE Eng Med Biol Soc. 2013;2013: 6671-6674.[31]Mi S,Connon CJ.The formation of a tissue-engineered cornea using plastically compressed collagen scaffolds and limbal stem cells.Methods Mol Biol. 2013;1014:143-155. [32]Hanazono Y,Takeda K,Niwa S,et al.Crystal structures of chitin binding domains of chitinase from Thermococcus kodakarensis KOD1.FEBS Lett.2016;590(2):298-304.[33]Zhao L,Wu Y,Chen S,et al.Preparation and characterization of cross-linked carboxymethyl chitin porous membrane scaffold for biomedical applications.Carbohydr Polym.2015;126: 150-155. [34]Abehsera S,Glazer L,Tynyakov J,et al.Binary gene expression patterning of the molt cycle: the case of chitin metabolism.PLoS One.2015;10(4):e0122602.[35]Alvarenga ES,Mansur JF,Justi SA,et al.Chitin is a component of the Rhodnius prolixus midgut.Insect Biochem Mol Biol. 2016; 69:61-70. [36]Kadokawa J,Endo R,Tanaka K,et al.Fabrication of porous chitin with continuous substructure by regeneration from gel with CaBr2•2H2O/methanol.Int J Biol Macromol. 2015;78: 313-317. [37]Zdarta J,Klapiszewski ?,Wysokowski M,et al. Chitin-lignin material as a novel matrix for enzyme immobilization.Mar Drugs.2015;13(4):2424-2446.[38]张卫.甲壳素及其衍生物共混膜的制备、性质和对创面的愈合作用研究[D].中国海洋大学,2013.[39]李玉文,张梁栋.甲壳素及其衍生物在医药领域的应用[J].潍坊学院学报,2011,11(4):80-84. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Li Qin, Mao Shuangfa, Li Min, Cheng Jiyan. Protective effect and mechanism of dendrobium on fibroblasts damaged by ultraviolet B [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1228-1233. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [6] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [7] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [8] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [9] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [10] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [11] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [12] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||