Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (33): 4985-4992.doi: 10.3969/j.issn.2095-4344.2016.33.017
Previous Articles Next Articles
Proteomics application progress in medical research
Li Yu-xiang, Rong Hao, Hu Qun-ying, Li Wen-hua
- Life Science Laboratory, School of Medicine, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China
-
Received:2016-06-08Online:2016-08-12Published:2016-08-12 -
Contact:Li Wen-hua, Professor, Master’s supervisor, Life Science Laboratory, School of Medicine, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China -
About author:Li Yu-xiang, Associate professor, School of Medicine, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China -
Supported by:the National Natural Science Foundation of China, No. 81360299; the Natural Science Foundation of Tibet, China, in 2015
CLC Number:
Cite this article
Li Yu-xiang, Rong Hao, Hu Qun-ying, Li Wen-hua. Proteomics application progress in medical research[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(33): 4985-4992.
share this article
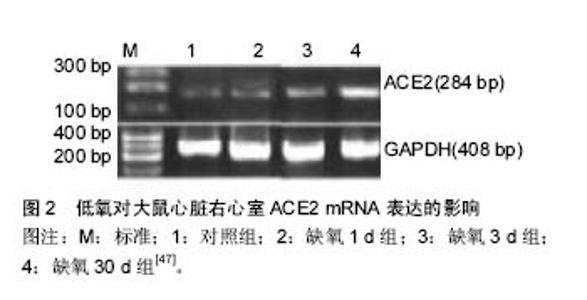
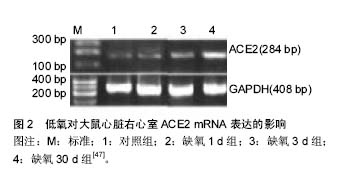
2.1 蛋白质组学的飞速发展 蛋白质组学是研究生物体、组织、细胞中所有蛋白质的组成、结构、功能及其蛋白相互作用的一门科学[2],其技术主要包括质谱技术、蛋白质芯片技术、双向电泳、表面等离子体共振技术、蛋白质复合物纯化技术和生物信息学分析等[16],近年来飞行质谱联用技术,具有大规模、高通量和系统化等特点及优势,在蛋白质组学研究中发挥着重要作用[17]。蛋白质的鉴定技术主要为质谱法(MS), 即利用样品离子化后, 离子间的质荷比(m/z)的差异来分析确定样品的分子质量现最常用的有两种,即基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF/MS)和电喷雾质谱(ESI-MS)[18]。 MALDI-TOF-MS是近年来应用较广,发展较快的软电离生物质谱,具有较高的灵敏度和分辨率,具有准确度高,重复性好,检测费用低等特点,已被广泛用于膀胱癌,胃癌,前列腺癌,肺癌,乳腺癌,卵巢癌,白血病,神经系统等疾病中,它与磁珠技术相结合,可有效地寻找出各类血清生物标志物,并在应用ClinProTools软件数据筛选出的蛋白质组可视化[19]。 血清蛋白组的研究内容大致可分为两个即:蛋白质表达模式的研究和蛋白质功能模式的研究。但是必须将血清进行特殊处理并结合多项技术才能很好的呈现蛋白质[20]。不同策略导致同样技术达成不同的结论和价值,同时海量数据并不能自然转化为知识或智慧。 蛋白质组是基因组的逻辑延伸,但研究策略是不同的,总结了4点关于蛋白质组学的研究策略:①以理解细胞内生命活动为出发点和目标的研究策略;②探寻蛋白质相互作用和调控的网络关系的研究策略;③高度重视动态研究及其策略。蛋白质组学已经成为细胞生物学的基础,动态和体内是蛋白质组学的目前趋势;④蛋白质组学干预与筛选交替进行的扇形拓展策略。此研究策略达到的效果:①有可能找到更多新的包括更好的标志蛋白;②有可能逐步拼出调控网络中的相关部分(一定意义上类似于测序的拼接) [9,21]。 2.2 蛋白质组学国内外研究现状 目前国内外蛋白质组学系列研究产生的数据将解读人类基因组这部“天书”,系统地揭示人体蛋白质组的组成、相互作用及其调节规律,书写后基因组研究篇章。将精确表达各种病理状态下蛋白质组的变化及其机制,构建基于人类蛋白质组的生理图谱和病理图谱,将揭示许多新的治疗靶点,新的诊断标志物和新的药物,为整体提升防治疾病水平提供新策略新手段。 2.2.1 蛋白质组学在恶性肿瘤研究中的应用 研究显示,基于质谱的蛋白质组技术广泛应用于癌症特异性生物标志物的研究。邓敬桓等[22]针对肝癌高发家族和无癌家族的患者的血清进行分析,得到血清蛋白质指纹图谱,再经过计算机进行分析,从而建立肝癌高发家族和无癌家族患者的比较筛选模型。王子赫等[23]认为基质辅助激光解析电离飞行时间质谱在肺癌早期诊断、筛查、疗效评价等方面有着良好的应用前景。范乃军等[24]应用弱阳离子磁珠联合基质辅助激光解吸离子化飞行时间质谱,建立胃癌与正常人血清蛋白质谱,共有48个差异蛋白峰,利用其中8个差异峰(质荷比分别为3 316.28,1 213.99,2 934.23,1 214.86,760.02,1 779.70,3 884.85及1 945.67)建立诊断胃癌模型。熊万成等[25]筛选出血清蛋白质谱中的29个显著差异蛋白质峰,据此建立的诊断乳腺癌模型。 综上所述,蛋白质组学在肝癌、肺癌、胃癌、乳腺癌、直肠癌、甲状腺癌、前列腺癌、等具有良好的应用前景。一方面,能筛选出相关的血清蛋白标志物,并建立相关疾病的诊断模型,提供一种全新的血清学诊断方法;另一方面,可通过这些特异性标志物,为易感人群的筛查、早期诊断、治疗选择和随访提供参考依据。 2.2.2 蛋白质组学在遗传性疾病中的应用 Yang等[26]应用MALDI-TOF MS/MS技术,鉴别出信号转导和细胞凋亡相关的肽基脯氨酸反式异构酶A和丝切蛋白-1,α-2-珠蛋白,分离出差异表达蛋白14种,建立大鼠阿尔兹海默病模型进行蛋白质组学的分析,有利于进一步深入了解阿尔兹海默病的发病机制。Rabilloud等[27]通过质谱技术和双向凝胶电泳,研究病态时和健康状态的人的线粒体蛋白质,可见疾病影响了核编码蛋白的稳定性。这种影响包括细胞色素C氧化酶亚单位的蛋白质表达水平下调。综上所述,蛋白质组学在遗传病,如遗传学球形红细胞增多症和阿尔兹海默病等,具有良好的应用前景。可通过蛋白质组学的技术,深入剖析这些遗传病的病理生理机制。在一定程度上,能增加对这些疾病的认识,也为疾病的预防和诊疗提供强有力的依据,寻找诊疗遗传病的生物标记物和关键的药物靶点。 2.2.3 蛋白质组学在药物研究中的应用 Li等[28]应用质谱技术和双向凝胶电泳,研究了导致神经退行性病变和记忆损伤发生的蓝藻细菌衍生物的微囊藻素-精氨酸-亮氨酸(MCRL)诱导神经毒性相关的蛋白发生改变所致。O'Connell等[29]通过采用液相色谱-质谱联用技术对3种前列腺癌细胞系,如DU145、22RV1和PC-3,与其对应的多西他赛抗性的子代细胞间进行比较分析,发现这有利于进一步了解其生化机制,提高临床诊疗效果。Jia等[30]通过采用2-D凝胶电泳和串联质谱技术,研究用乙醇诱导的肝硬化模型,发现16中差异蛋白可能成为酒精性肝硬化的新药靶点。研究表明,利用蛋白质组学技术寻找新药物的靶点是一把利器。 2.2.4 蛋白质组学在微生物学研究中的应用 基于质谱的蛋白质组技术应用于微生物学特异性生物标志物的研究。Fernandez等[31]通过采用双向凝胶电泳和MALDI-TOF MS技术,分析羧甲基纤维素培养的葡萄胞菌蛋白质中分离出的蛋白斑点,发现诸多蛋白质促使其发挥致病作用。Fang等[32]通过采用2-DE和MALDI-TOF MS技术,研究草莓幼苗叶子,发现凯尔文循环、糖酵解途径受阻和49就差异表达蛋白,使得了解其病原抗性机制。Ansong等[33]通过液相色谱-质谱联用技术,分别对对数期、静止期、和在低pH/低Mn条件下的沙门菌属的蛋白质进行分析,发现高表达S.thphi Ty2蛋白,而这蛋白可能与宿主和S.thphi的病原性特异性相关。综上所述,蛋白质组学在微生物学研究中发挥着重要的作用,其研究深入到生命科学的诸多领域,一方面,有利于研究病原微生物的病原抗性机制、致病机制等;另一方面,有利于研制疫苗,促进健康。 2.2.5 蛋白质组学在高原医学研究中的应用 血清蛋白质组学分析系统包括质谱系统、磁珠分离系统、可选的体液样品自动处理系统和分析软件。将收集的患者或正常对照组的血浆[34]、血清[35]、脑脊髓液[36]、尿液等样本[37];磁珠分离去除样本中的其他杂质和高丰度蛋白,如盐等,收集低丰度目的蛋白;加入基质混合后,直接点在AnchorChip靶上,进行飞行时间质谱分析;通过软件筛选患者或正常对照组的差异表达蛋白,获得两者的特异质谱图谱。该研究方法目前主要应用于肿瘤标志物的筛选与诊断[38-40]。由于高原习服过程中,机体会发生严重的生理生化的变化,在很大程度上会反映在血清的组份变化上,预期会有很好的研究和应用前景。 近年来学者已经意识到蛋白质组学在高原医学 研究中的应用是一新的技术手段,目前蛋白质组学应用于高原医学研究中,有些聚焦在发现高原低氧适应机制方面,如:低氧大鼠海马CA3区和CA1区蛋白质组学研究中[41],99个蛋白质点被鉴定,其中15个蛋白质点表达有明显差异多位于CA1区,相关的蛋白可能有细胞新陈代谢相关蛋白和结构蛋白以及凋亡相关蛋白,低氧导致CA1区损伤发生的机制可能与应激蛋白增强、新陈代谢增强和细胞凋亡有关,为了解神经组织适应低氧环境的分子机制和提高神经组织低氧的耐受性提供帮助。有些研究采用质谱技术联合双向电泳,比较平原地区的藏族、世居高原藏族和低海拔地区的尼泊尔世居者的肌肉蛋白质组,7个差异蛋白质被鉴定,研究显示藏族高海拔世居者和低海拔地区的藏民可能存在某些防止氧化性损伤机制[42]。进行质谱技术和双向电泳分析基因敲除肌红蛋白的小鼠对低氧环境的良好适应,显示其脂肪酸连接蛋白和热休克蛋白27表达下降,可能是肌肉蛋白质组脂肪能量代谢变化所致[43]。 为了研究急进高原时习服的规律和机制,作者课题组将平原雄性SD大鼠运往高原,光、电镜观察大鼠心脏、肺脏、脑组织,应用 RT-PCR、Western Blot、实时定量PCR,系统观察了不同高原实地低氧暴露时间对相关基因表达蛋白的影响. 将60只随机分为6 组,每组10只,分别为高原低氧1,2,3,7,30 d组和对照组(西安地区,海拔5 m)。高原低氧1 d 组由西安地区途中耗时1 d带到海拔2 700 m青海格尔木地区,高原低氧2 d组途中耗时2 d带到海拔5 000 m唐古拉地区,高原低氧3,7,30 d组途中耗时3 d带到海拔4 500 m西藏那曲地区,7 d组在那曲饲养7 d。30 d组在那曲饲养30 d。经过高原低氧习服后心肺组织病变明显减轻[44-45],同时可以观察到HIF-1 mRNA表达水平逐渐升高[46];缺氧30 d组右心室明显代偿性肥大伴右心功能显著上调,同时右心室血管紧张素转换酶2(ACE2)mRNA、蛋白合成均有明显增加(P < 0.01)[47](如图2)。脑组织热休克蛋白70(Hsp70)生成量与高原暴露时间成正比[48]。不同基因型的大鼠其血浆肾素,血管紧张素Ⅱ,醛固酮的浓度变化存在差异,ACE2基因型为GG的,血浆肾素,血管紧张素Ⅱ含量显著上升,醛固酮含量明显下降(P < 0.01)。ACE2 基因型AA个体血浆肾素、ATⅡ含量上升,醛固酮含量下降(P < 0.05),而ACE2基因型AG血浆肾素、血管紧张素Ⅱ,醛固酮变化不明显(P > 0.05)[49]。从平原进入高原,机体从整体、器官、细胞及分子水平等不同层次适应高原低氧环境。其中HIF-1α在细胞水平低氧应答反应中起核心作用,直接或间接的控制着百余种基因,包括细胞生长,凋亡,能量代谢,血管生成等基因的表达。肾素-血管紧张素-醛固酮系统(RAAS)是整体水平低氧应答反应的重要环节,在高原低氧习服中发挥着重要的作用。"
| [1] He F. Human liver proteome project: plan, progress, and perspectives. Mol Cell Proteomics.2005;4(12): 1841-1848. [2] Kahn P.From gnnome to proteorae:looking at a cell’s proteins Science.1995;270(5235):369-370. [3] Elzek MA,Rodland KD.Proteomics of ovarian cancer: functional insights and clinical applications. Cancer and Metastasis Reviews.2015;34(1): 83-96. [4] Ebhardt HA,Root A,Sander C,et al. Applications of targeted proteomics in systems biology and translational medicine. Proteomics.2015;15(18): 3193-3208. [5] Yan X,Yuan F,Chen X,et al. Bioinformatics analysis to identify the differentially expressed genes of glaucoma. Molecular Medicine Reports.2015;12:4829-4836. [6] Simonson TS,McClain DA,Jorde LB,et al. Genetic determinants of Tibetan high-altitude adaptation. Human Genetics.2012;131:527-533. [7] Ji LD,Qiu YQ,Xu J,et al. Genetic Adaptation of the Hypoxia-Inducible Factor Pathway to Oxygen Pressure among Eurasian Human Populations. Molecular Biology And Evolution.2012;29:3359-3370. [8] Ge RL,Simonson TS,Cooksey RC,et al.Metabolic insight into mechanisms of high-altitude adaptation in Tibetans. Molecular Genetics And Metabolism.2012; 106:244-247. [9] 李文华,刘忠,袁东亚,等.高原分子医学[M].上海:复旦大学出版社,2011:12. [10] Richalet JP.Cardiopulmonary Adaptation to High Altitude.Cardiac Adaptations. Springer New York.2013: 233-249. [11] Hussey D, Koul P, Prchal JT. High Altitude Genetic Adaptation In Tibetans Does Not Include Increased Hemoglobin-Oxygen Affinity. Blood.2013;122(21): 937-937. [12] Luks AM, Hackett PH.High Altitude and Common Medical Conditions.High Altitude. Springer New York. 2014:449-477. [13] Yi X, Liang Y, Huerta-Sanchez E, et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science.2010;329:75-78. [14] DeLellis SM, Anderson SE, Lynch JH, et al. Acute Mountain Sickness Prophylaxis: A High-Altitude Perspective. Current sports medicine reports.2013; 12(2):110-114. [15] Carter EA, Mayo JR, MacInnis MJ, et al.Individual Susceptibility to High Altitude and Immersion Pulmonary Edema and Pulmonary Lymphatics. Aviation.Space. and Environmental Medicine.2014; 85(1): 9-14. [16] 尹稳,伏旭,李平.蛋白质组学的应用研究进展[J].生物技术通讯, 2014,(1):32-38. [17] 高雪,郑俊杰,贺福初.我国蛋白质组学研究现状及展望[J]. 生命科学, 2007,19(3):257-263. [18] Picotti P, Bodenmiller B, Aebersold R. Proteomics meets the scientific method. Nature Methods.2013;10: 24-27. [19] Peng M, Taouatas N, Cappadona S, et al. Protease bias in absolute protein quantitation. Nature Methods. 2012;9:524-525. [20] Allison Doerr. Mass spectrometry–based targeted proteomics.Nature Methods.2013;10:23. [21] Altelaar AF, Munoz J, Heck AJ.Next-generation proteomics: towards an integrative view of proteome dynamicsNature Reviews Genetics. 2013;14,35-48. [22] 邓敬桓,陈智平,李山,等. 应用SELDI-TOF-MS技术筛选肝癌遗传性血清蛋白标志物[J].世界华人消化杂志, 2014, 5: 018. [23] 王子赫,刘晓晴. MALDI-TOF质谱技术在肺癌中的应用[J].国际肿瘤学杂志, 2014, 41(5): 344-347. [24] 范乃军,魏东,赵艇.应用磁珠联合质谱技术建立胃癌诊断模型[J].中华实验外科杂志, 2014, 31(11):2559-2561. [25] 熊万成,高春芳,范乃军.应用液体蛋白芯片-飞行时间质谱系统研究乳腺癌血清差异蛋白表达[J].河南科技大学学报:医学版, 2014,32 (2): 89-93. [26] Yang H, Qiao H, Tian X. Proteomic analysis of cerebral synaptoso mes isolated from rat model of alzheimer’s disease. Indian J Exp Biol.2011;49(2):118-124. [27] Rabilloud T, Sturb JM, Carte N, et al.Comparative proteomics as a new tool for exploring human mitochondrial tRNA disorders. Biochemistry.2002; 41(1):144-150. [28] Li G, Cai F, Yan W, et al. A proteomic analysis of MCLR-induced neurotoxicity :implications for Alzheimer’s disease. Toxicol Sci.2012;127(2):485-495. [29] O’Connell K,Prencipe M,O'Neill A,et al.The use of LC-MS to identify differentially expressed proteins in docetaxel-resistant prostate cancer cell lines. Proteomics.2012;12(13):2115-2126. [30] Jia XF, Yin L, Feng YL, et al. A dynamic plasma membrane proteome analysis of alcohol-induced liver cirrhosis. Proteome Science.2012;10:39. [31] Fernández-Acero FJ, Colby T,Harzen A,et al.Proteomic analysis of the phytopathogenic fungus Botrytis cinerea during cellulose degradation.Proteomics. 2009;9(10): 2892-2902. [32] Fang X, Chen W, Xin Y, et al. Proteomic analysis of strawberry leaves infected with Colletotrichum fragariae. J Proteomics.2012;75(13):4074-4090. [33] Carter EA, Mayo JR, MacInnis MJ, et al.Individual Susceptibility to High Altitude and Immersion Pulmonary Edema and Pulmonary Lymphatics. Aviation, Space. and Environmental Medicine.2014;85(1): 9-14. [34] Solier C, Langen H. Antibody‐based proteomics and biomarker research-Current status and limitations. Proteomics.2014;14(6): 774-783. [35] Gaudet P, Michel PA, Zahn-Zabal M, et al.The neXtProt knowledgebase on human proteins: current status. Nucleic acids research.2015;43(D1): D764-D770. [36] Gopal J,Muthu M,Chun SC,et al. State-of-the-art nanoplatform-integrated MALDI-MS impacting resolutions in urinary proteomics.PROTEOMICS-Clinical Applications.2015;9(5-6):469-481. [37] Almeida AM, Bassols A, Bendixen E, et al. Animal board invited review: advances in proteomics for animal and food sciences. Animal.2015;9(1):1-17. [38] Zeidan BA, Townsend PA, Garbis SD,et al.Clinical proteomics and breast cancer. The Surgeon.2015; 13(5):271-278. [39] Garcia-Santamarina S,Boronat S,Dombneeh A,et al.Monitoring in vivo reversible cysteine oxidation in proteins using ICAT and mass spectrometry.Nat Protoc.2014;9(5):1131-1145. [40] 赵倩倩,张自强,李岩.质谱技术在肿瘤蛋白质标志物研究中的应用与发展[J].中华检验医学,2015,38(2):82-84. [41] Carter EA, Mayo JR, MacInnis MJ, et al. Individual Susceptibility to High Altitude and Immersion Pulmonary Edema and Pulmonary Lymphatics. Aviat Space Environ Med. 2014;85(1):9-14. [42] Fulco CS, Beidleman B A, Muza SR. Effectiveness of Preacclimatization Strategies for High-Altitude Exposure. Exerc Sport Sci Rev. 2013;41(1):55-63.. [43] Richalet JP. Cardiopulmonary Adaptation to High Altitude.Cardiac Adaptations. Springer New York.2013: 233-249. [44] Li WH, Liu ZH, Yuan DY,et al. Plateau molecular medicine[M]. Fudan University press.2011:12. [45] Simonson TS,McClain DA,Jorde LB,et al. Genetic determinants of Tibetan high-altitude adaptation. Human Genetics.2012;131: 527-533. [46] 李文华,袁东亚,孙芳云,等. 高原低氧环境对大鼠肺组织影响[J].中国公共卫生,2012,28(7): 945-948. [47] Li WH, Liu Z.High Altitude Hypoxia Environment changes of the Content of RAAS and right ventricular ACE2 activity in adult SD rats.IEEE.2011;(10): 8300-8302. [48] Li WH,Yuan DY,Zhang M,et al. Altitude hypoxia on the ultrastructure of rat brain and Hsp70 expression.Amino Acids.2011;7:S20-S21. [49] 李文华,刘忠.高原低氧环境下大鼠RAAS水平与ACE2基因多态性[J].现代预防医学,2011,38(15):2944-2946. [50] 罗勇军,陈郁,高钰琪.高原肺水肿的血浆蛋白质组学研究[J].解放军医学杂志, 2012, 37(1): 31-33. [51] 张元元,段瑞峰,汪海.高原脑水肿患者血浆的蛋白质组学研究[J].中国应用生理学杂志,2011,27(2):180-184. [52] 刘琦,刘新宇,张仁卿,等.汉,藏族肺结核患者血清差异蛋白质的质谱分析[J].第三军医大学学报, 2010, 32(18): 1986-1990. [53] 闫成,薛改,吴丽颖,等.两种肝组织匀浆上清液诱导人脐带间充质干细胞向肝细胞分化的比较[J].中国组织工程研究, 2015,19(19): 2993-2998. [54] 丁金萍,刘宏伟,晏丹,等.应用比较蛋白质组学技术筛查组织工程化软骨力学性能相关蛋白[J].中华整形外科杂志, 2013,29(001): 49-54. [55] 郭宜姣,李文华.骨缺损修复生物工程研究进展[J].中国骨质疏松杂志,2014,20(8):988-993. [56] Yang J,Li WH,Liu S,et al. Identification of novel serum peptide biomarkers for high-altitude adaptation: a comparative approach. Sci Rep. 2016;6:25489. [57] Shirato K,Nakajima K,Korekane H,et al. Hypoxic regulation of glycosylation via the N-acetylglucosamine cycle. J Clin Biochem Nutr. 2011;48(1):20-25. [58] Belo AI,van Vliet SJ,Maus A,et al. Hypoxia inducible factor 1α down regulates cell surface expression of α1,2‐fucosylated glycans in human pancreatic adenocarcinoma cells. FEBS letters. 2015;589(18): 2359-2366. [59] Ferrer CM,Lynch TP,Sodi VL,et al. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Molecular Cell. 2014; 54(5): 820-831. |
| [1] | Gu Xia, Zhao Min, Wang Pingyi, Li Yimei, Li Wenhua. Relationship between hypoxia inducible factor 1 alpha and hypoxia signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1284-1289. |
| [2] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [3] | Zhao Xiang, Wei Cuilan, Zhang Yeting. Neurogenesis and neuroinflammation under exercise: alteration and regulation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 813-820. |
| [4] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [5] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [6] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| [7] | Yang Junhui, Luo Jinli, Yuan Xiaoping. Effects of human growth hormone on proliferation and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3956-3961. |
| [8] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [9] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [10] | Li Xinping, Cui Qiuju, Zeng Shuguang, Ran Gaoying, Zhang Zhaoqiang, Liu Xianwen, Fang Wei, Xu Shuaimei. Effect of modification of β-tricalcium phosphate/chitosan hydrogel on growth and mineralization of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3493-3499. |
| [11] | Zhou Anqi, Tang Yufei, Wu Bingfeng, Xiang Lin. Designing of periosteum tissue engineering: combination of generality and individuality [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3551-3557. |
| [12] | Chen Song, He Yuanli, Xie Wenjia, Zhong Linna, Wang Jian. Advantages of calcium phosphate nanoparticles for drug delivery in bone tissue engineering research and application [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3565-3570. |
| [13] | Lin Haiqi, Chen Liang, Tang Lu, Weng Xiquan, Lin Wentao. Significance of urinary proteomics assessing pathological changes in the body [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3259-3266. |
| [14] | Dai Min, Wang Shuai, Zhang Nini, Huang Guilin, Yu Limei, Hu Xiaohua, Yi Jie, Yao Li, Zhang Ligang. Biological characteristics of hypoxic preconditioned human amniotic mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3004-3008. |
| [15] | Li Xiangze, Bu Xianmin, Li Dongmei, Chi Yulei, Su Qiang, Jin Xintong, Zhao Jian, Zhang Gaotian, Wu Bin, Meng Chunyang . Stem cells, cytokines, hormones, neuropeptides and genes in traumatic brain trauma to promote fracture healing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3057-3063. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||