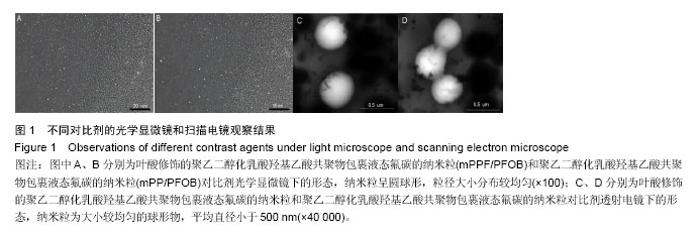
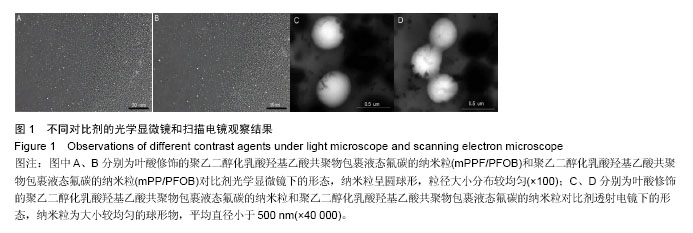
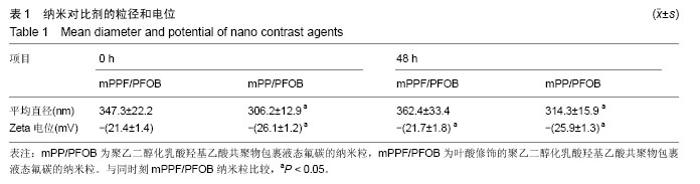
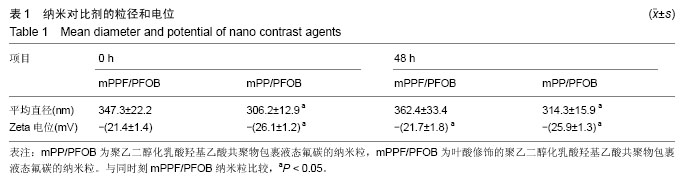
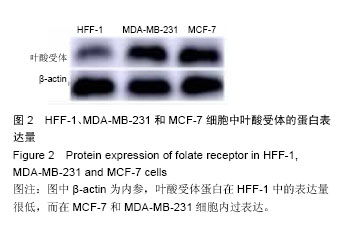
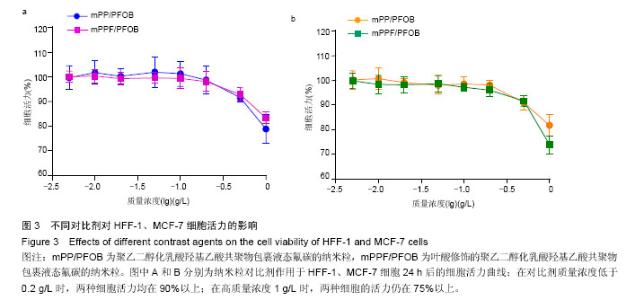
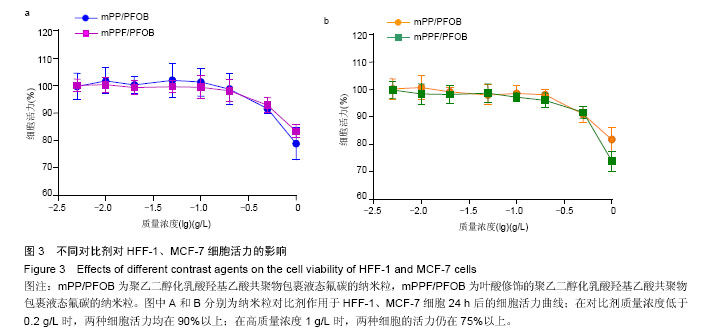
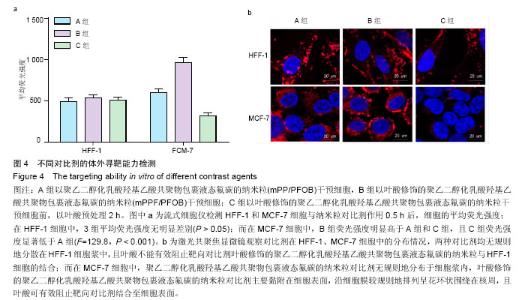
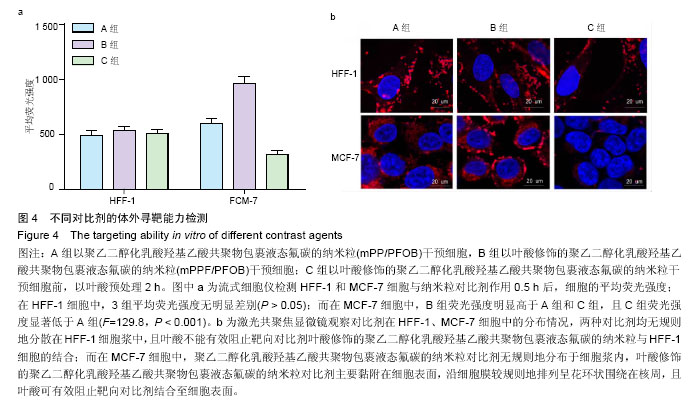
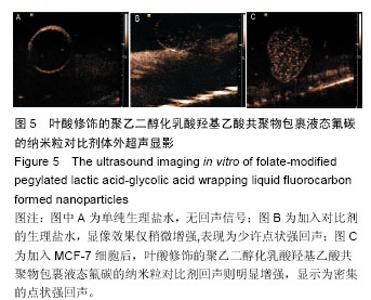
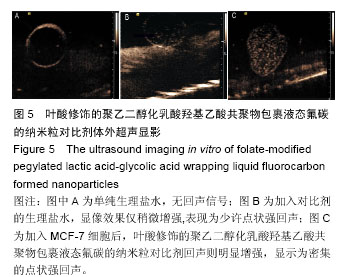
| [1] Siegel RL,Miller KD,Jemal A.Cancer statistics,2015.CA Cancer J Clin. 2015;65(1):5-29.
[2] Wenhua D,Lijia L,Hui W,et al.The clinical significance of real-time contrast-enhanced ultrasonography in the differential diagnosis of breast tumor. Cell Biochem Biophys. 2012;63(2):117-120.
[3] Zhao H,Xu R,Ouyang Q,et al.Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol. 2010;73(2): 288-293.
[4] Saracco A,Szabó BK,Aspelin P,et al.Differentiation between benign and malignant breast tumors using kinetic features of real-time harmonic contrast-enhanced ultrasound.Acta Radiol. 2012;53(4): 382-388.
[5] Hu Q,Wang XY,Zhu SY,et al.Meta-analysis of contrast-enhanced ultrasound for the differentiation of benign and malignant breast lesions.Acta Radiol. 2015; 56(1):25-33.
[6] Morawski AM,Lanza GA,Wickline SA.Targeted contrast agents for magnetic resonance imaging and ultrasound.Curr Opin Biotechnol.2005;16(1):89-92.
[7] Della-Longa S,Arcovito A.Structural and functional insights on folate receptor alpha (FRalpha) by homology modeling, ligand docking and molecular dynamics.J Mol Graph Model.2013;44:197-207.
[8] Necela BM,Crozier JA,Andorfer CA,et al.Folate receptor-alpha (FOLR1) expression and function in triple negative tumors.PLoS One. 2015;10(3):e0122209.
[9] Cai HH,Pi J,Lin X,et al.Gold nanoprobes-based resonance Rayleigh scattering assay platform: Sensitive cytosensing of breast cancer cells and facile monitoring of folate receptor expression.Biosens Bioelectron.2015;74:165-169.
[10] Siafaka P,Betsiou M,Tsolou A,et al.Synthesis of folate- pegylated polyester nanoparticles encapsulating ixabepilone for targeting folate receptor overexpressing breast cancer cells.J Mater Sci Mater Med.2015;26(12):275.
[11] Kurosaki A,Hasegawa K,Kato T,et al.Serum folate receptor alpha (FRA) as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression.Int J Cancer. 2016;138(8):1994-2002.
[12] Elnakat H,Ratnam M.Role of folate receptor genes in reproduction and related cancers.Front Biosci. 2006;11: 506-519.
[13] Driver BR,Barrios R,Ge Y,et al.Folate Receptor alpha Expression Level Correlates With Histologic Grade in Lung Adenocarcinoma.Arch Pathol Lab Med. 2015. [Epub ahead of print]
[14] Zhao H,Yung LY.Selectivity of folate conjugated polymer micelles against different tumor cells.Int J Pharm.2008;349(1-2):256-268.
[15] Liu H,Shen M,Zhao J,et al.Facile formation of folic acid-modified dendrimer-stabilized gold-silver alloy nanoparticles for potential cellular computed tomography imaging applications.Analyst. 2013; 138(7):1979-1987.
[16] Zhang L,Zhu D,Dong X,et al.Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery.Int J Nanomedicine. 2015;10: 2101-2114.
[17] Liu J,Qin Y,Li D,et al.Highly sensitive and selective detection of cancer cell with a label-free electrochemical cytosensor.Biosens Bioelectron. 2013;41:436-441.
[18] Hu Y,Ma B,Zhang Y,et al.Small molecule-folic acid modification on nanopatterned PDMS and investigation on its surface property.Biomed Microdevices. 2014;16(3):487-497.
[19] Sega EI,Low PS.Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev.2008;27(4):655-664.
[20] Kalber TL,Kamaly N,So PW,et al.A low molecular weight folate receptor targeted contrast agent for magnetic resonance tumor imaging.Mol Imaging Biol. 2011;13(4):653-662.
[21] Barar J,Kafil V,Majd MH,et al.Multifunctional mitoxantrone-conjugated magnetic nanosystem for targeted therapy of folate receptor-overexpressing malignant cells. J Nanobiotechnology.2015;13:26.
[22] Assaraf YG,Leamon CP,Reddy JA.The folate receptor as a rational therapeutic target for personalized cancer treatment.Drug Resist Updat.2014;17(4-6):89-95.
[23] Semete B,Booysen L,Lemmer Y,et al.In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems.Nanomedicine. 2010;6(5): 662-671.
[24] Afshari M,Derakhshandeh K,Hosseinzadeh L.Characterisation, cytotoxicity and apoptosis studies of methotrexate-loaded PLGA and PLGA-PEG nanoparticles.J Microencapsul.2014;31(3):239-245.
[25] Zhou W,Zhou Y,Wu J,et al.Aptamer-nanoparticle bioconjugates enhance intracellular delivery of vinorelbine to breast cancer cells.J Drug Target. 2014; 22(1):57-66.
[26] Sun Y,Zhu Y,Huang C,et al.Magnetite loaded Polypeptide-PLGA multifunctional microbubbles for dual-mode US/MR imaging.Contrast Media Mol Imaging. 2016;11(2):146-153.
[27] Rapoport N,Gao Z,Kennedy A.Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy.J Natl Cancer Inst.2007; 99(14): 1095-106.
[28] 凌友,黄岳山,梁常艳,等.叶酸修饰乳酸-羟基乙酸共聚物纳米粒的制备及性能表征[J].中国组织工程研究与临床康复,2008,12(36):7049-7052.
[29] 陈澄,宫玉萍,王志刚,等.制备包裹吲哚菁绿和液态氟碳的纳米级双模态造影剂[J].中国医学影像技术, 2015,31(2): 186-190.
[30] Rogalska A,Szwed M,Rychlik B.The connection between the toxicity of anthracyclines and their ability to modulate the P-glycoprotein-mediated transport in A549, HepG2, and MCF-7 cells.ScientificWorldJournal. 2014;2014:819548.
[31] Toy R,Bauer L,Hoimes C,et al.Targeted nanotechnology for cancer imaging. Adv Drug Del Rev. 2014;76:79-97.
[32] 田慧玲,周凤华,郑洁,等.Ki-67、AR及FRA在三阴性乳腺癌中的表达及意义[J].现代肿瘤医学, 2014,22(4): 830-833. |