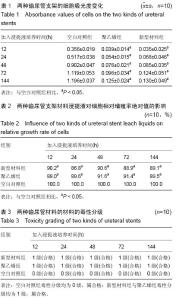
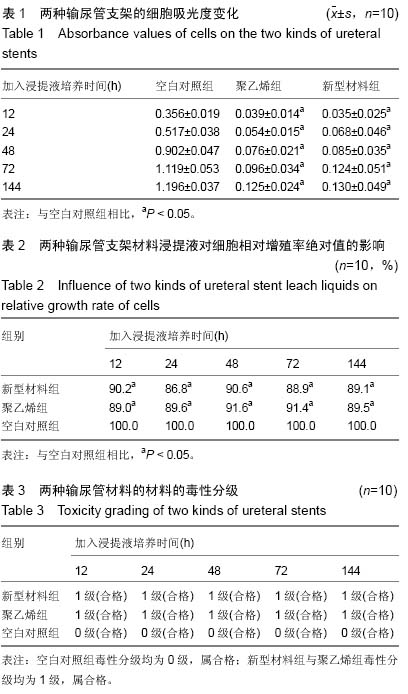
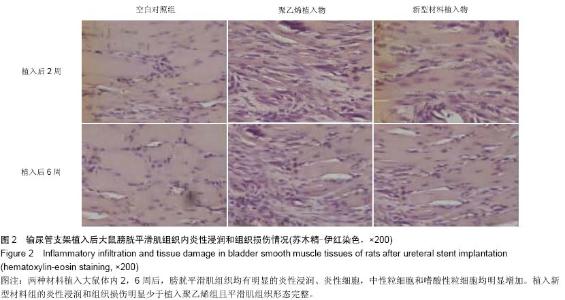
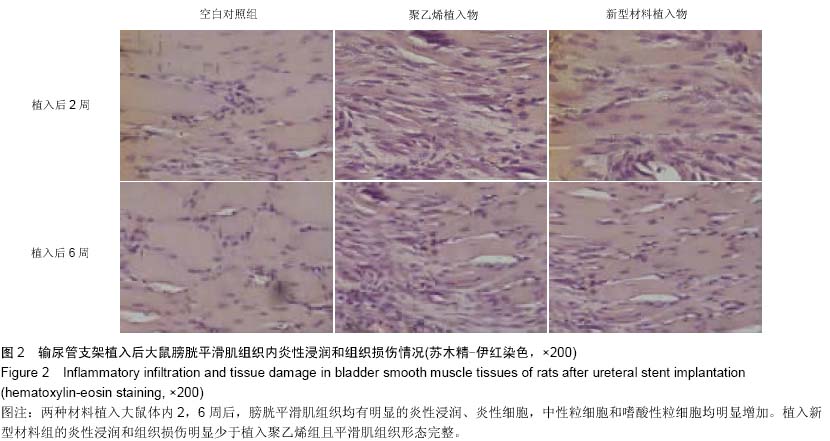
Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (25): 3996-4001.doi: 10.3969/j.issn.2095-4344.2015.25.012
Previous Articles Next Articles
Liu Min, Master, Associate chief physician, Department of Urology, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai 201700, China
Liu Min1, Yan Wei1, Li Hui-feng1, Zhu Tong-yu2
- 1Department of Urology, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai 201700, China;
2Department of Urology, Zhongshan Hospital Affiliated to Fudan University, Shanghai 200032, China
-
Online:2015-06-18Published:2015-06-18 -
Contact:Zhu Tong-yu, M.D., Professor, Chief physician, Department of Urology, Zhongshan Hospital Affiliated to Fudan University, Shanghai 200032, China -
About author:Liu Min, Master, Associate chief physician, Department of Urology, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai 201700, China
CLC Number:
Cite this article
Liu Min, Yan Wei, Li Hui-feng, Zhu Tong-yu. Liu Min, Master, Associate chief physician, Department of Urology, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai 201700, China[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(25): 3996-4001.
share this article
| [1] Demanes DJ, Banerjee R, Cahan BL, et al. Ureteral stent insertion for gynecologic interstitial high-dose-rate brachytherapy. Brachytherapy. 2015;14(2):245-251. [2] Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12(1):17-25. [3] Kumar S, Shankaregowda SA, Devana SK, et al. Single-incision multiport laparoendoscopic technique to repair retrocaval ureter using the Santosh PGI ureteric tacking fixation technique. Asian J Endosc Surg. 2014;7(4):337-341. [4] Halder P, Shukla RM, Mandal KC, et al. Double obstruction of ureter: a diagnostic challenge. J Indian Assoc Pediatr Surg. 2014;19(3):129-132. [5] Wang J, Feng J, Hu W, et al. Preclinical evaluation of a newly designed ureteral stent and magnetic retrieval catheter for minimally invasive stent removal. Urology. 2014;84(4): 960-966. [6] You JH, Kim YG, Kim MK. Should we place ureteral stents in retroperitoneal laparoscopic ureterolithotomy? Consideration of surgical techniques and complications. Korean J Urol. 2014; 55(8):511-514. [7] Loh-Doyle JC, Low RK, Monga M, et al. Patient experiences and preferences with ureteral stent removal. J Endourol. 2015; 29(1):35-40. [8] Selli C, Turri FM, Gabellieri C, et al. Delayed-onset ureteral lesions due to thermal energy: an emerging condition. Arch Ital Urol Androl. 2014;86(2):152-153. [9] Zou T, Wang L, Li W, et al. A resorbable bicomponent braided ureteral stent with improved mechanical performance. J Mech Behav Biomed Mater. 2014;38:17-25. [10] Manabe N, Kirikoshi R, Takahashi O. Glycolic Acid-Catalyzed Deamidation of Asparagine Residues in Degrading PLGA Matrices: a Computational Study. Int J Mol Sci. 2015;16(4): 7261-7272. [11] Yada S, Terakawa M. Femtosecond laser induced periodic surface structure on poly-L-lactic acid. Opt Express. 2015;23(5):5694-5703. [12] Huang D, Li D, Wang T, et al. Isoniazid conjugated poly(lactide-co-glycolide): Long-term controlled drug release and tissue regeneration for bone tuberculosis therapy. Biomaterials. 2015;52:417-425. [13] Manassero F, Ortori S, Gabellieri C, et al.An unusual case of intrarenal coiled and ruptured guidewire. Arch Ital Urol Androl. 2015;87(1):90-92. [14] Adanur S, Aydin HR, Ozkaya F, et al. Holmium laser lithotripsy with semi-rigid ureteroscopy: a first-choice treatment for impacted ureteral stones in children? Med Sci Monit. 2014;20: 2373-239. [15] Park HK, Paick SH, Kim HG, et al. The impact of ureteral stent type on patient symptoms as determined by the ureteral stent symptom questionnaire: a prospective, randomized, controlled study. J Endourol. 2015;29(3):367-371. [16] García-Aparicio L, Blázquez-Gómez E, Martin O, et al. Bacterial characteristics and clinical significance of ureteral double-J stents in children. Actas Urol Esp. 2015;39(1): 530-536. [17] Vogt B, Desgrippes A, Desfemmes FN. Pigtail suture stent: decisive progress towards double-pigtail stent tolerance and unexpected properties of the suture in the ureter. Prog Urol. 2014;24(7):441-450. [18] Georgescu D, Mul?escu R, Geavlete B, et al. Ureteroscopy-first-line treatment alternative in ureteral calculi during pregnancy? Chirurgia (Bucur). 2014;109(2):229-232. [19] Shaheen T, Edirisinghe T, Gabriel M, et al. In vitro encrustation of a semi-permanent polymer-covered nitinol ureter stent: an artificial urine model. Urolithiasis. 2014; 42(3):203-207. [20] Choi H, Kim JH, Park JY, et al. A modified laparoscopic ureterolithotomy by pulling ureter with Carter-Thomason fascial closure and ureter incision by broken 15th blade. Scand J Surg. 2014;103(3):195-200. [21] Chung HH, Kim MD, Won JY et al. Multicenter experience of the newly designed covered metallic ureteral stent for malignant ureteral occlusion: comparison with double J stent insertion. Cardiovasc Intervent Radiol. 2014;37(2):463-470. [22] Beraldo S, Neubeck K, Von Friderici E, et al.The prophylactic use of a ureteral stent in laparoscopic colorectal surgery. Scand J Surg. 2013;102(2):87-89. [23] Rabani SM. Combined percutaneous and transurethral lithotripsy for forgotten ureteral stents with giant encrustation. Nephrourol Mon. 2012;4(4):633-635. [24] Lock JY, Wyatt E, Upadhyayula S, et al. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications. J Biomed Mater Res A. 2014;102(3):781-792. [25] Pettenati C, El Fegoun AB, Hupertan V et al. Double J stent reduces the efficacy of extracorporeal shock wave lithotripsy in the treatment of lumbar ureteral stones. Cent European J Urol. 2013;66(3):309-313. [26] Roy N, Waingankar S, Aggarwal G.Assessment of bio-safety of low-cost polyurethane urologic stents used in developing countries. Indian J Urol. 2012;28(2):184-188. [27] Kawahara T, Ito H, Terao H, et al. Changing to a loop-type ureteral stent decreases patients' stent-related symptoms. Urol Res. 2012;40(6):763-767. [28] Ulvik O, Ulvik NM. Diversity in urologists' personal preferences in the ureteroscopic management of ureteral calculi in Norway. Scand J Urol. 2013;47(2):126-130. [29] Coccolini F, Lotti M, Manfredi R, et al. Ureteral stenting in cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy as a routine procedure: evidence and necessity. Urol Int. 2012;89(3):307-310. [30] Vahidi B, Fatouraee N. A biomechanical simulation of ureteral flow during peristalsis using intraluminal morphometric data. J Theor Biol. 2012;298:42-50. [31] Greaves NS, Iqbal SA, Hodgkinson T, et al. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen. 2015. [32] Barygina V, Becatti M, Lotti T, et al. Treatment with low-dose cytokines reduces oxidative-mediated injury in perilesional keratinocytes from vitiligo skin. J Dermatol Sci. 2015. [33] Aziz S, Ahmed SS, Ali A, et al. Salivary immunosuppressive cytokines IL-10 and IL-13 are significantly elevated in oral squamous cell carcinoma patients. Cancer Invest. 2015. [34] Peng Z, Chen W, Wang L, et al. Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int J Clin Exp Pathol. 2015 Mar 1;8(3):2680-2689. [35] Nielsen KR, Steffensen R, Haunstrup TM, et al. Inherited variation in immune response genes in follicular lymphoma and diffuse large b-cell lymphoma. Leuk Lymphoma. 2015: 1-66. [36] Kulcsar KA, Baxter VK, Abraham R, et al. Distinct immune responses in resistant and susceptible strains of mice during neurovirulent alphavirus encephalomyelitis. J Virol. 2015. [37] Ampel NM, Nesbit LA, Nguyen CT, et al. Cytokine profiles from antigen-stimulated whole blood samples among patients with pulmonary and non-meningeal disseminated coccidioidomycosis. Clin Vaccine Immunol. 2015. [38] Cardoso CP, Oliveira AJ, Botoni FA, et al. Interleukin-10 rs2227307 and CXCR2 rs1126579 polymorphisms modulate the predisposition to septic shock. Mem Inst Oswaldo Cruz. 2015. [39] Xiong J, Wang Y, Zhang Y, et al. Lack of Association between interleukin-10 gene polymorphisms and graft rejection risk in kidney transplantation recipients: a meta-analysis.PLoS One. 2015;10(6):e0127540. [40] Santer P, Pejo E, Feng Y, et al. Cyclopropyl-methoxycarbonyl Metomidate: Studies in a Lipopolysaccharide Inflammatory Model of Sepsis. Anesthesiology. 2015. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||