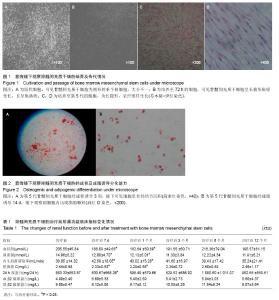
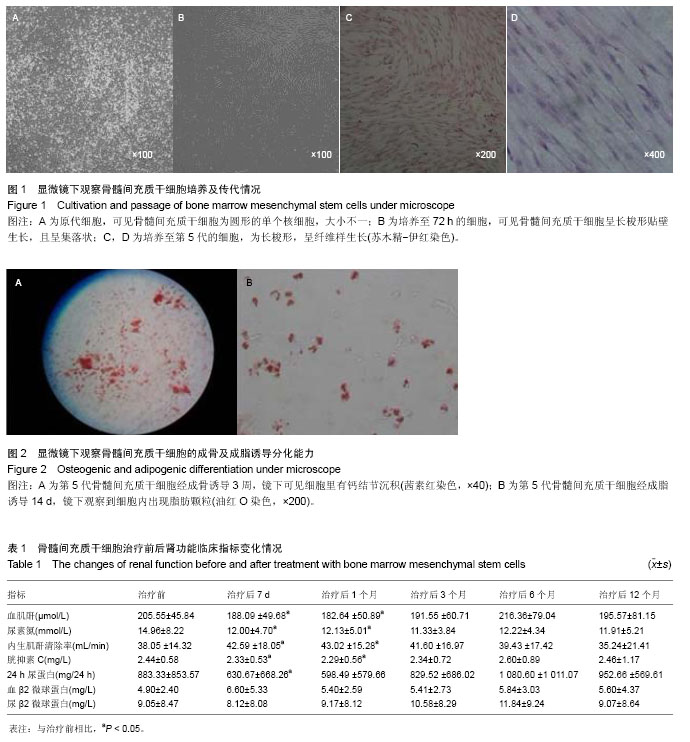
| [1] Pascual J, Pérez-Sáez MJ, Mir M,et al. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando). 2012;26(4):280-290.
[2] Le Blanc K, Rasmusson I, Sundberg B,et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363 (9419):1439-1441.
[3] García-Olmo D, García-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation.Dis Colon Rectum. 2005;48(7):1416-1423.
[4] Bang OY, Lee JS, Lee PH,et al.Autologous mesenchymal stem cell transplantation in stroke patients.Ann Neurol. 2005; 57(6):874-882.
[5] Chen S, Liu Z, Tian N,et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery.J Invasive Cardiol. 2006;18(11): 552-556.
[6] Karantalis V, DiFede DL, Gerstenblith G,et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial.Circ Res. 2014; 114(8):1302-1310.
[7] 王平贤.慢性移植肾肾病[J].中华器官移植杂志,2005,25(5): 318-320.
[8] 潘兴华,张步振,庞荣清.干细胞—人类疾病治疗的新希望[M].云南:云南科学技术出版社,2004:120-121.
[9] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells.Science. 1999;284(5411):143-147.
[10] Klyushnenkova E, Mosca JD, Zernetkina V,et al.T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression.J Biomed Sci. 2005;12(1):47-57.
[11] Ninichuk V, Gross O, Segerer S,et al.Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice.Kidney Int. 2006;70(1):121-129.
[12] Herrera MB, Bussolati B, Bruno S, et al. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury.Int J Mol Med. 2004;14(6):1035-1041.
[13] Prodromidi EI, Poulsom R, Jeffery R,et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome.Stem Cells. 2006;24(11):2448-2455.
[14] Cornell LD, Colvin RB.Chronic allograft nephropathy.Curr Opin Nephrol Hypertens. 2005;14(3):229-234.
[15] Braun C, Schultz M, Fang L, et al.Treatment of chronic renal allograft rejection in rats with a low-molecular-weight heparin (reviparin).Transplantation. 2001;72(2):209-215.
[16] Klyushnenkova E, Mosca JD, Zernetkina V,et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression.J Biomed Sci. 2005;12(1):47-57.
[17] Tan J, Wu W, Xu X,et al.Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307(11):1169-1177. |