Chinese Journal of Tissue Engineering Research
Previous Articles Next Articles
Entecavir combined with immunoglobulin prevents hepatitis B recurrence after liver transplantation
Gao Yin-jie, Liu Zhen-wen, Zhang Min, Su Hai-bin, Zhou Shuang-nan, Zhou Xia, Zhang Da-li, He Xi, Tang Ru-jia
- Liver Transplantation Center, 302nd Hospital of PLA, Beijing 100039, China
-
Received:2012-10-16Revised:2013-01-14Online:2013-07-30Published:2013-07-30 -
Contact:Liu Zhen-wen, Master, Chief physician, Liver Transplantation Center, 302nd Hospital of PLA, Beijing 100039, China 13911395948@163.com -
About author:Gao Yin-jie★, Master, Attending physician, Liver Transplantation Center, 302nd Hospital of PLA, Beijing 100039, China gaoyj302@163.com
CLC Number:
Cite this article
Gao Yin-jie, Liu Zhen-wen, Zhang Min, Su Hai-bin, Zhou Shuang-nan, Zhou Xia, Zhang Da-li, He Xi, Tang Ru-jia. Entecavir combined with immunoglobulin prevents hepatitis B recurrence after liver transplantation[J]. Chinese Journal of Tissue Engineering Research, doi: 10.3969/j.issn.2095-4344.2013.31.002.
share this article

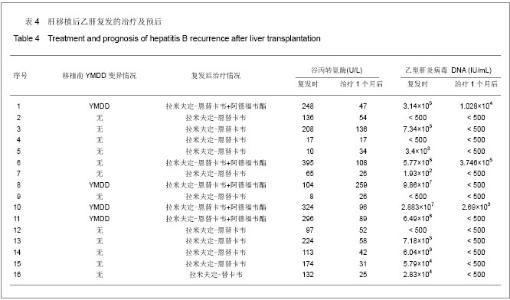
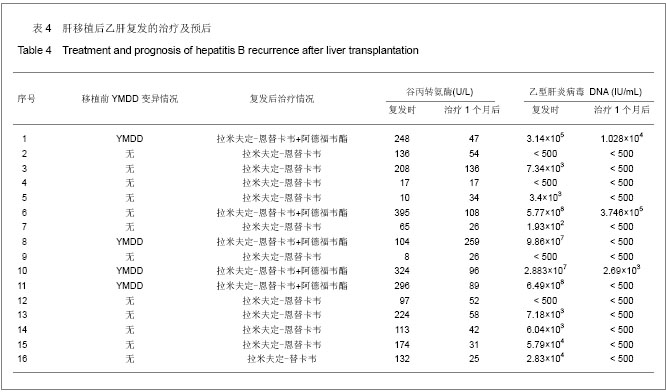
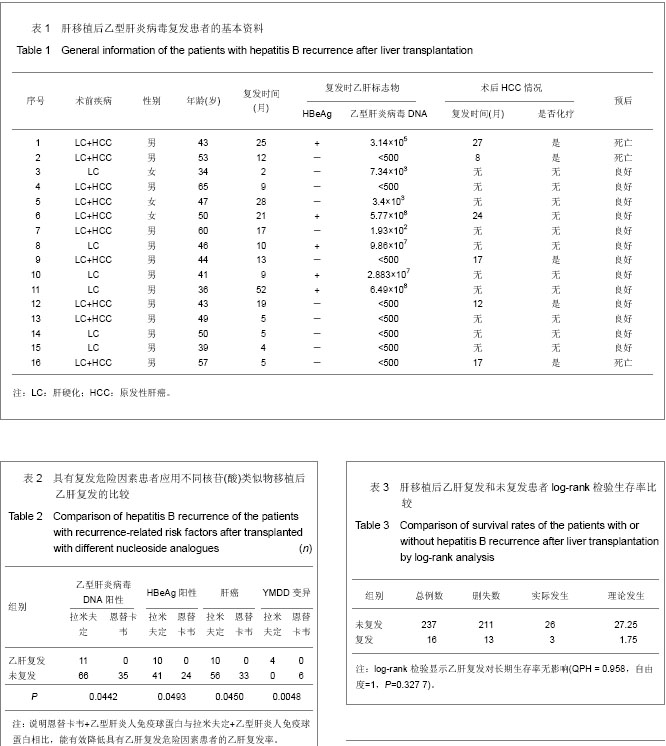
2.1 参与者数量分析 纳入患者253例,按意向性处理分析,全部进入结果分析。 2.2 乙型肝炎病毒相关性肝移植患者生存分析 253例乙型肝炎病毒相关性肝移植患者共死亡29例,死亡率为11.46%(29/253)。肝移植后6个月内死亡13例,12个月内死亡17例,24个月内死亡24例,36个月内死亡25例,48个月内死亡28例,60个月内29例。死亡原因中肝癌复发21例,原发性肝无功3例,胆道并发症2例,多器官功能衰竭1例,脑干出血1例,呼吸系统衰竭1例。6年生存曲线见图1。 2.3 拉米夫定+乙型肝炎人免疫球蛋白与恩替卡韦+乙型肝炎人免疫球蛋白预防乙肝复发的疗效比较 253例患者中应用拉米夫定+乙型肝炎人免疫球蛋白预防组202例,平均年龄(48.04±8.72)岁,平均随访(61.8±18.3)个月,有16例复发,16例复发患者复发时资料见表1。复发时间为(14.625±14.778)个月,复发率为7.92% (16/202),平均复发时间(18.42±17.04)个月,死亡26例。肝移植后各时相点乙型肝炎病毒累积复发率为见图2。"
肝移植后半年复发率为2.08%(5/240),肝移植后1年为3.81%(9/236),肝移植后2年为5.68%(13/229),肝移植后3年为6.58%(15/228),肝移植后4年为6.67%(15/225),肝移植后5年为7.14%(16/224)。恩替卡韦+乙型肝炎人免疫球蛋白组51例,平均年龄(46.96±6.64)岁,平均随访时间(57.1±15.9)个月,未见复发,死亡3例。二者复发率(P=0.079 3)、生存率(P=0.248 5)比较差异均无显著性意义,说明恩替卡韦+乙型肝炎人免疫球蛋白与拉米夫定+乙型肝炎人免疫球蛋白相比,均能有效预防肝移植后乙肝复发。 对于具有乙肝复发危险因素(移植前HBeAg阳性、乙型肝炎病毒DNA阳性、肝癌、YMDD变异)的患者[6-10],经统计学分析,恩替卡韦+乙型肝炎人免疫球蛋白较拉米夫定+乙型肝炎人免疫球蛋白肝移植后乙肝复发率显著较少,差异有显著性意义,见表2。 "
| [1] Todo S, Demetris AJ, Van Thiel D, et al.Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology.1991; 13(4):619-626.[2] 黄洁夫.中国肝脏移植[M].北京:人民卫生出版社,2008:676-678.[3] Takaki A, Yagi T, Iwasaki Y, et al. Short-term high-dose followed by long-term low-dose hepatitis B immunoglobulin and lamivudine therapy prevented recurrent hepatitis B after liver transplantation.Transplantation.2007;83(2):231-233.[4] 中华医学会肝病学分会,中华医学会感染病学分会.慢性乙型肝炎防治指南(2010版)[J].临床肝胆病杂志,2011,27(1):Ⅰ-ⅩⅥ.[5] 沈中阳.肝脏移植术后乙肝复发的综合防治[J].中国医学科学院报,2005,27(4):431-434.[6] Rosenaul J, MatnⅡas J,Hans L, et al.Lamivudine and low--dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection.Hepatologv.2001;34:895-902.[7] Kiyici M,Yilmaz M,Akyildiz M,et al.Association between hepatitis B and hepatocellular carcinoma recurrence in patients undergoing liver transplantation. Transplant Proc. 2008;40: 1511-1517.[8] Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology 2000;32:1189-1195.[9] Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842-1847.[10] Terrault N, Roche B, Samuel D. Management of the hepatitis B virus in the liver transplantation setting: a European and an American perspective. Liver Transpl. 2005;11:716-732.[11] Omata M. Significance of extra hepatic replication of hepatitis B virus.Hepatol. 1990; 12 (2): 364-366.[12] Fang Q, Zhao LS, Zhou TY, et al.Reinfection of HBV and its possible mechanism in patients with liver transplantation. Chin J Hepatol. 2005;13 (3): 225-227.[13] Joya-Vazquez PP, Dodson FS, Dvorchik I, et al. Impact of anti-hepatitis B c-positive grafts on the outcome of liver transplantation for HBV-related cirrhosis. Transplantation. 2002;73:1598-1602.[14] Doson SF, Issa S, Araya V, et al. Infectivity of hepatitis allografts with antibodies to hepatitis B virus. Transplantation. 1997; 11:1582-1584.[15] Murray KF, Carithers RL, Jr, AASLD. Practice Guidelines: Evaluation of the Patient for Liver Transplantation. Hepatology. 2005;41(6):1407-1432.[16] Lok AS, Heathcote EJ, Hoofnagle JH.Management of hepatitis B: summary of a workshop.Gastroenterology.2001; 120(7):1828-1853.[17] Saab S, Kim M, Wright TL, et al. Successful orthotopic liver transplantation for lamivudine-associated YMDD mutant hepatitis B virus. Gastroenterology. 2000;119(5): 1382-1384.[18] Wong SN, Chu CJ, Wai CT, et al. Low risk of hepatitis B virus recurrence after withdrawal of long-term hepatitis B Immunoglobulin in patients receiving maintenance nucleoside analogue therapy. Liver Transpl. 2007; 13(3): 374-381.[19] Seehofer D. Preoperative antiviral treatment and postoperative prophyaxis in HBV DNA positive patients undergoing liver transplantation.Liver Transpl. 2001; 72:1381-1385.[20] European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol.2009;50:227-242.[21] Liaw YF,Leung N,Kao JH,et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int.2008;2:263-283.[22] 郑俊福,韩大康,李丽,等.恩替卡韦联合乙型肝炎免疫球蛋白预防肝移植术后乙型肝炎再感染[J].世界华人消化杂志,2009,17(7): 716-719.[23] 蔡常结,赵辉,郭怡,等.恩替卡韦预防肝移植术后乙型肝炎病毒再感染的临床研究[J].中华普通外科文献:电子版,2008,2(5): 385-389.[24] Samuelson A, Morgan M, Reynolds J. The use of entecavir following liver transplantation: pilot safety and tolerability data. Am J Gastroenterol. 2008;103:S160.[25] Samuel D, Forns X, Berenguer M, et al. Report of the monothematic EASL conference on liver transplantation for viral hepatitis (Paris, France, January 12-14, 2006). Hepatology. 2006;45: 127-143.[26] Chang TT, Gish RG, de Man R,et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med.2006; 354: 1001-1010.[27] Lai CL, Rosmawati M, Lao J, et al. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology. 2002;123: 1831-1838.[28] Tenney DJ, Levine SM, Rose RE, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother.2004;48:3498-3507.[29] Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology.2009;49:1503-1514.[30] Fontana RJ, Hann HW, Wright T, et al. A multicenter study of lamivudine treatment in 33 patients with hepatitis B after liver transplantation. Liver Transpl.2001;7:504-510.[31] Mutimer D, Feraz-Neto BH, Harrison R, et al. Acute liver graft failure due to emergence of lamivudine resistant hepatitis B virus: rapid resolution during treatment with adefovir. Gut. 2001;49:860-863.[32] Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg- negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011- 1020.[33] Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg positive chronic hepatitis B. N Engl J Med. 2006;354: 1001-1010.[34] Marion P, Hann HW, Paul M, et al.Adefovir Dipivoxil (ADV) Alone And In Combination With Lamivudine (LAM) Suppresses YMDD Mutant Hepatitis B Virus Replication: 48 Week Preliminary Analysis. Abstract 845. 53rd AASLD. 2002.Boston, MA. Hepatology.2002;36(Sup 1):6-7. |
| [1] | Yang Xin, Jin Zhe, Feng Xu, Lu Bing. The current situation of knowledge and attitudes towards organ, eye tissue, body donation of residents in Shenyang [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 779-784. |
| [2] | Wang Yanjiao, Wang Rui, Sun Luning. Bankart pepair versus Bristow-Latarjet procedure for recurrent anterior instability of the shoulder: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(21): 3423-3430. |
| [3] | Tian Lin, Shi Xiaoqing, Mao Jun, Zhang Nongshan, Zhang Li, Xing Runlin, Wang Peimin. Meta-analysis of vacuum-sealing drainage combined with antibiotic bone cement in the treatment of chronic osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2618-2624. |
| [4] | Li Liqiang, Jiao Longxing, Zhang Wu, Yan Wentao, Li Jian, Li Minghao. Effect of immature dendritic cells derived from bone marrow on rejection of orthotopic liver transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2025-2029. |
| [5] | Qian Yuzhang, Wang Nan, Dong Yuqi, Xie Lin, Kang Ran. Factors for the recurrence of lumbar disc herniation after percutaneous transforaminal endoscopic discectomy: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(36): 5886-5896. |
| [6] | Yang Feng, Chang Lipu, Huang Changshan, Gong Xiaoguang, Chang Shunwu. Macrolide antibiotics protects against ischemia-reperfusion injury after liver transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(26): 4176-4182. |
| [7] | Liu Zhiling, Gao Minghong, Chen Yingxin. Bio-engineering cornea versus human donor cornea in the treatment of fungal corneal ulcer [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(10): 1563-1569. |
| [8] | Liu Mengyuan1, Fang Fang2. Risk factors for multi-drug resistant organisms infection after liver transplantation [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(7): 1109-1114. |
| [9] | Pan Lijuan, Wang Rongli. Therapeutic effect of autologous source induced pluripotent stem cell transplantation on chronic hepatitis B-induced liver injury mice [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(5): 773-778. |
| [10] | Wang Zhen, Zhang Xuejun, Chen Yuqing, Zhang Lei, Zhu Zunmin, Li Yulong, Huang Zhoufeng, Lian Cheng, Shi Mingyue, Zhang Hongsheng, Wang Fuxu, Wen Shupeng, Yang Jing, Zheng Meiqiong, Sun Kai. Allogeneic chimeric antigen receptor T-cell therapy for recurrent chronic myeloid leukemia in lymphoid blast crisis after allogeneic hematopoietic stem cell transplantation: a two-case report and literature review [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(33): 5263-5268. |
| [11] | Gao Hongqiang, Liu Jing, Li Zhiqiang, Wang Hailei, Zhao Xiongqi, Zhang Shengning, Ran Jianghua, Li Li . Ulinastatin improves rat liver metabolism after reduced-size liver transplantation [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(3): 435-440. |
| [12] | Cai Qiucheng, Fan Hongkai, Xiong Rihui, Jiang Yi. Intravenous administration of bone marrow mesenchymal stem cells protects liver function following fatty liver transplantation from donors after cardiac death [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(17): 2625-2629. |
| [13] | Jiang Shanshan, Wang Feng, Yu Limei. Immunomodulatory properties of mesenchymal stem cells and their application in organ transplantation [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(1): 103-109. |
| [14] | Wan Ding-ming, Sun Lin-lin, Xie Xin-sheng, Guo Rong, Cao Wei-jie, Zhang Su-ping, Li Li, Chen Xiao-na, Liu Yu-ye. Peripheral blood haploidentical hematopoietic stem cell transplantation for treatment of acute lymphoblastic leukemia in adults: monitoring minimal residual diseases to intervene recurrence [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(9): 1413-1418. |
| [15] | Ren Hao, Dong Jing, Liu Li-wei, Chen Zhao-lin, Pan Jin-jin, Chen Xi, Song Hai-yan, Zhang Jun-fei, Chen Cong-xin, Liu Bo. Risk factors for hepatocellular carcinoma in hepatitis B cirrhosis patients given treatment with human umbilical cord mesenchymal stem cell [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(9): 1350-1356. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||