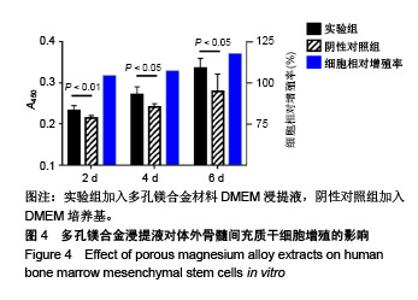
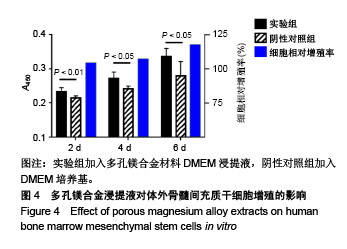
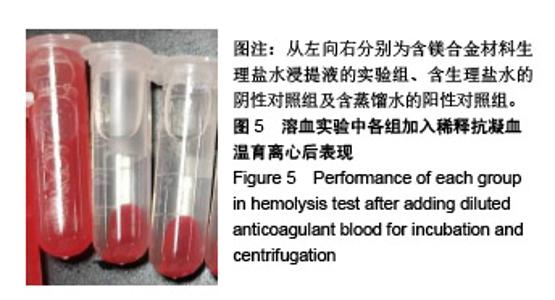
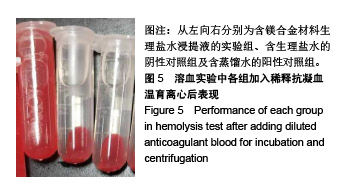
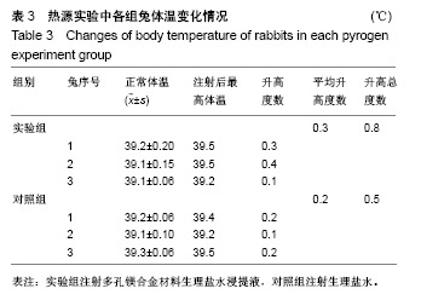
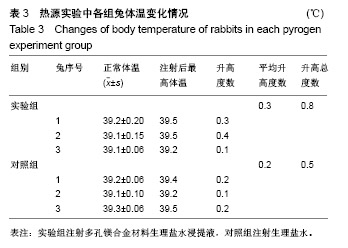
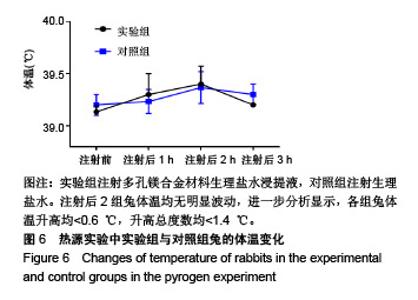
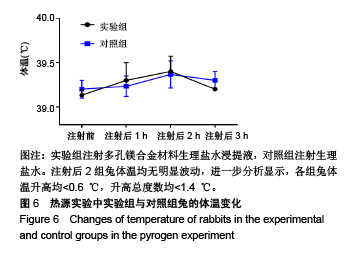
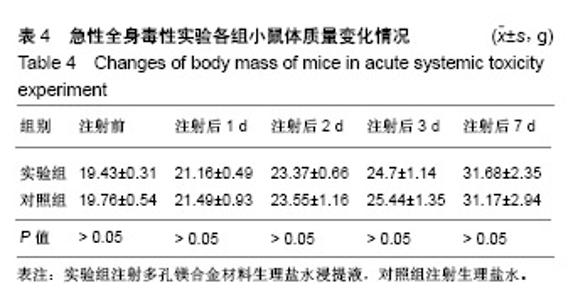
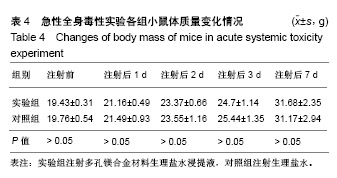
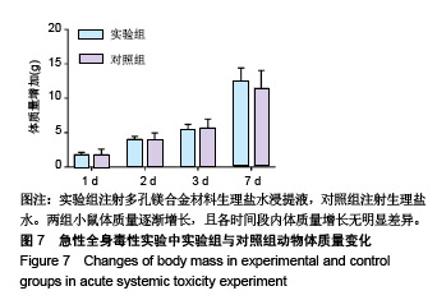
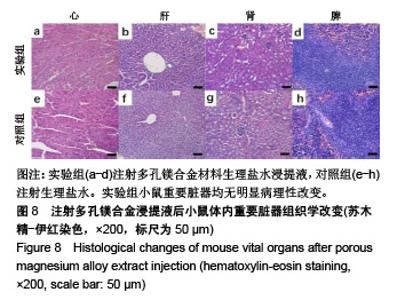
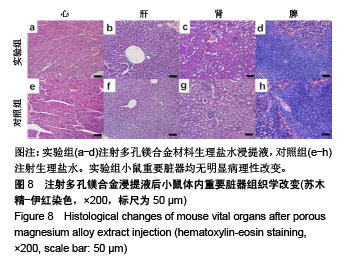
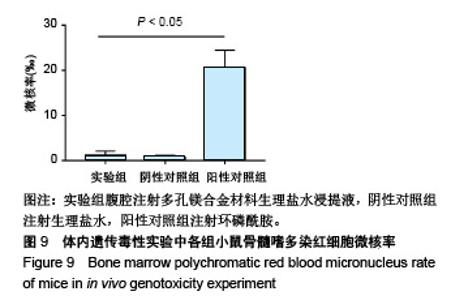
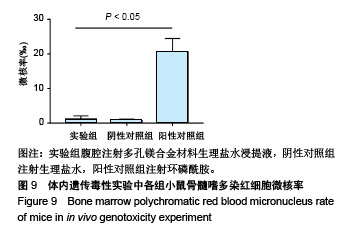
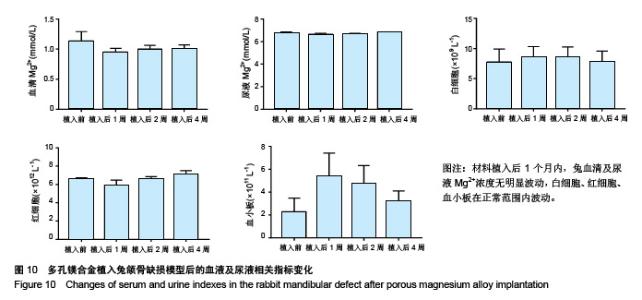
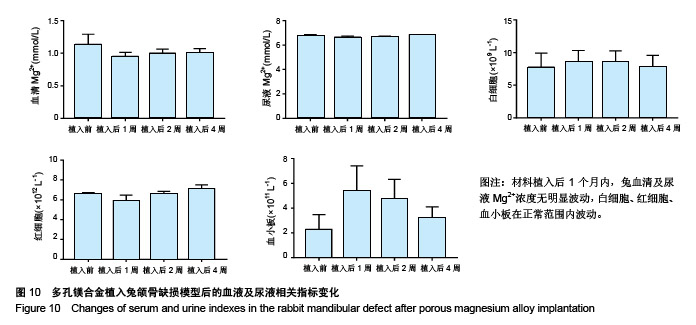
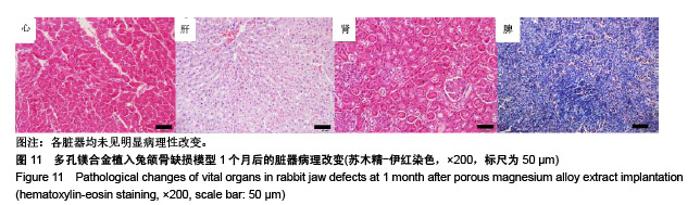
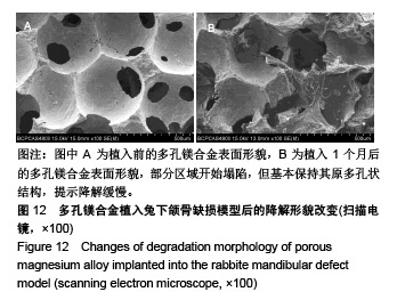
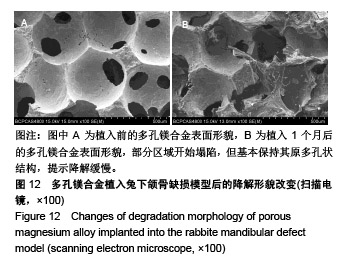
| [1]成翔宇,纪斌,庞金辉.骨折的生物学内固定及内固定材料性能分析[J].中国组织工程研究, 2012,16(22):4121-4124.[2]厉国定,胡晓亮,倪明,等.生物可吸收材料在骨折内固定治疗的临床研究[J].生物骨科材料与临床研究, 2011,8(1):34-35,38.[3]Niu J,Yuan G,Liao Y,et al.Enhanced biocorrosion resistance and biocompatibility of degradable Mg-Nd-Zn-Zr alloy by brushite coating.Mater Sci Eng C Mater Biol Appl. 2013;33(8): 4833-4841.[4]Mao L,Shen L,Chen J,et al.Enhanced bioactivity of Mg-Nd-Zn-Zr alloy achieved with nanoscale MgF2 surface for vascular stent application.ACS Appl Mater Interfaces. 2015; 7(9):5320-5330.[5]袁广银,章晓波,牛佳林,等.新型可降解生物医用镁合金JDBM的研究进展[J].中国有色金属学报, 2011,21(10):2476-2488.[6]Chen Y,Zhao S,Liu B,et al.Corrosion-controlling and osteo-compatible Mg ion-integrated phytic acid (Mg-PA) coating on magnesium substrate for biodegradable implants application.ACS Appl Mater Interfaces. 2014;6(22): 19531-19543.[7]Wang Y,Ouyang Y,Pang X,et al.Effects of degradable MG-ND-ZN-ZR alloy on osteoblastic cell function.Int J Immunopathol Pharmacol.2012;25(3):597-606.[8]Guan X,Xiong M,Zeng F,et al.Enhancement of osteogenesis and biodegradation control by brushite coating on Mg-Nd-Zn-Zr alloy for mandibular bone repair.ACS Appl Mater Interfaces.2014;6(23):21525-21533.[9]Yoshizawa S,Brown A,Barchowsky A,et al.Role of magnesium ions on osteogenic response in bone marrow stromal cells.Connect Tissue Res.2014;55 Suppl 1:155-159.[10]孔祥东,郝永强,王磊,等.新型医用可降解镁合金(JDBM)螺钉的生物安全性研究[J].组织工程与重建外科杂志, 2015,11(3): 124-127.[11]Jia G,Hou Y,Chen C,et al.Precise fabrication of open porous Mg scaffolds using NaCl templates: Relationship between space holder particles, pore characteristics and mechanical behavior.Mater Design.2018;140:106-113.[12]奚廷斐.医疗器械生物学评价标准[M].北京:中国标准出版社, 2012.[13]陈硕,蒋电明,何彬,等.新型自组装肽纳米纤维支架材料的生物安全性评价[J].中国现代医学杂志, 2017,27(1):16-23.[14]Yu Y,Lu H,Sun J.Long-term in vivo evolution of high-purity Mg screw degradation - Local and systemic effects of Mg degradation products.Acta Biomater.2018;71:215-224.[15]Windhagen H,Radtke K,Weizbauer A,et al.Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online.2013;12:62.[16]Lee JW,Han HS,Han KJ,et al.Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy.Proc Natl Acad Sci U S A.2016;113(3):716-721.[17]曹耿华,毕衍泽,陈民芳,等.骨折内固定用新型Mg-Zn-Ca/HA复合材料的成分、组织结构与性能[J].稀有金属材料与工程, 2013, 42(8):1632-1637.[18]Jiang H,Wang J,Chen M,et al. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo.Mater Sci Eng C Mater Biol Appl.2017;75:1068-1074.[19]曲立杰,李慕勤,刘苗,等.超声-微弧氧化医用镁合金体外降解性研究[Z].西安:96-100.[20]Zhao C,Hou P,Ni J,et al.Ag-Incorporated FHA Coating on Pure Mg: Degradation and in Vitro Antibacterial Properties. ACS Appl Mater Interfaces.2016;8(8):5093-5103.[21]马越,程翔,曹尤雅,等.牙科用微弧氧化Zr-Cu-Al-Ag合金的血液相容性评价[J].现代口腔医学杂志, 2016,30(6):352-355.[22]朱兆金.骨科新型医用可降解植入材料JDBM镁合金的生物毒性、髓内针及植入物感染细菌生物膜的基础研究[D].苏州:苏州大学,2013.[23]刘天甲,顾硕,周璐,等.可降解镁合金颅骨固定系统在动物实验中的安全性及有效性[J].中国组织工程研究, 2018,22(30): 4876-4881.[24]Qin H,Zhao Y,An Z,et al.Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy.Biomaterials.2015;53:211-220.[25]邵惠锋,贺永,傅建中.增材制造可降解人工骨的研究进展—从外形定制到性能定制[J].浙江大学学报(工学版), 2018,52(6): 1035-1057.[26]倪昕晔,熊信柏,尤瑞金,等.碳/碳复合材料表面掺镁羟基磷灰石生物涂层的体外性能[J].中国组织工程研究, 2017,21(34): 5443-5448.[27]Zhao SF,Jiang QH,Peel S,et al.Effects of magnesium-substituted nanohydroxyapatite coating on implant osseointegration.Clin Oral Implants Res.2013;24 Suppl A100:34-41.[28]王平,张会敏,李刚. 鹿角多肽对骨髓间充质干细胞的影响[J].中华中医药杂志,2018,33(12):5644-5647.[29]Wang Y,Zhu Z,He Y,et al.In vivo degradation behavior and biocompatibility of Mg-Nd-Zn-Zr alloy at early stage.Int J Mol Med.2012;29(2):178-184.[30]章晓波,毛琳,袁广银,等.心血管支架用Mg-Nd-Zn-Zr生物可降解镁合金的性能研究[J].稀有金属材料与工程, 2013,42(6): 1300-1305.[31]洪岩松,杨柯,张广道,等.可降解镁合金的动物体内骨诱导作用[J].金属学报,2008,44(9):1035-1041. |