Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (10): 1611-1616.doi: 10.3969/j.issn.2095-4344.1620
Previous Articles Next Articles
Application of chitosan hydrogels in cartilage repair
Yang Lingjian, Li Yanlin, Jia Di, He Yinghong, Xiang Yaoyu
- Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650000, Yunnan Province, China
-
Received:2018-11-13 -
Contact:Li Yanlin, Doctoral supervisor, Chief physician, Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650000, Yunnan Province, China -
About author:Yang Lingjian, Master candidate, Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650000, Yunnan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81460340 and 81760403 (both to LYL)
CLC Number:
Cite this article
Yang Lingjian, Li Yanlin, Jia Di, He Yinghong, Xiang Yaoyu. Application of chitosan hydrogels in cartilage repair[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1611-1616.
share this article
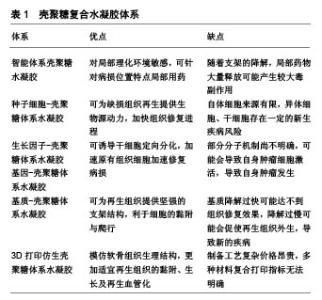
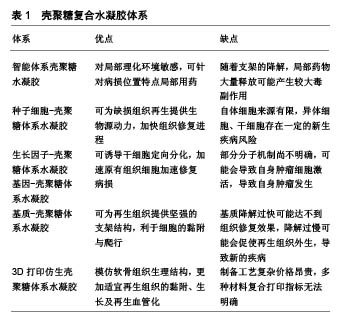
壳聚糖复合水凝胶主要有智能体系壳聚糖水凝胶、种子细胞-壳聚糖体系水凝胶、生长因子-壳聚糖体系水凝胶、基因-壳聚糖体系水凝胶、基质-壳聚糖体系水凝胶、3D打印仿生壳聚糖体系水凝胶,各类壳聚糖复合水凝胶体系的优点及不足,见表1。 2.1 智能体系壳聚糖水凝胶 智能型壳聚糖水凝胶是一类对外界物理/化学环境变化敏感、随着环境变化而自身理化性质发生改变的智能组织工程材料[9],大致可分为:pH敏感型、温度敏感型、电场敏感型、光敏感型、磁场敏感型和多重敏感型。智能型壳聚糖水凝胶具有以下优点:①可模拟细胞外基质环境,给组织修复提供支架和细胞生长微环境[2];②以半溶液性质载药,药物在局部温度的影响下形成水凝胶,靶向治疗且避免药物过快流失;③与不同化合物通过物理/化学方法结合,构成三维网状交联结构,形成新兴组织工程修复支架等[10]。温敏型、磁场敏感型水凝胶在关节软骨修复中被广泛研究,其中温敏型水凝胶尤为突出,其余智能型水凝胶在软骨修复中尚未报道。 2.1.1 温敏型壳聚糖水凝胶 Chenite等[11]最早发现单独壳聚糖溶液不具备温敏性,而在β-甘油磷酸钠干预下,壳聚糖/β-甘油磷酸钠溶液在一定温度时发生凝胶化。其原理可能为壳聚糖/β-甘油磷酸钠溶液在达到临界温度时,壳聚糖分子上氨基发生去质子化,分子间静电作用降低,同时分离的质子与β-甘油磷酸钠羟基相结合,疏水基团凝聚形成分子内氢键,发生凝胶化[12]。由于壳聚糖/β-甘油磷酸钠温敏性凝胶依靠分子内氢键及分子间作用力相连接,故存在其稳定性欠佳、容易快速降解及药物半衰期短等缺点[13]。Song等[14]将壳聚糖/β-甘油磷酸酯/明胶混合构建水凝胶,当其处于生理体温37 ℃时发生凝胶化,扫描电镜发现水凝胶结构稳定;体外联合培养脂肪干细胞并诱导成软骨分化,干细胞在5 d内表现高生存力形态;体内实验显示,诱导分化细胞在凝胶支架材料维持下可增殖更多同种细胞,并且细胞外基质产生一定血管化。虽然此类温敏性凝胶结构稳定,但机械性能较差,抗阻抗旋转等方面有所欠缺,未来研究可进一步改善。 Deng等[15]利用NaHCO3作为偶联剂,发现随着NaHCO3浓度的增加,水凝胶凝胶化时间缩短,机械强度增强,但高渗性溶液会引起细胞溶解。若将150 mL 50%β-甘油磷酸钠溶于0.4 mol/L NaHCO3与壳聚糖溶液中制备温敏性凝胶,可缩短凝胶化时间,并且热稳定性和机械强度符合人体膝关节内负荷,同时可将细胞毒性降至最低,扫描电镜观察水凝胶呈现不规则粗糙多孔形态,有利于细胞的附着和生长,为进一步研究提供了借鉴。 随着研究的不断推进,壳聚糖温敏水凝胶有望制备成为可注射型试剂,在体外物理诱导条件下将药物局部定点传递至病变部位,在减轻全身药物毒性反应的同时也达到了精准医疗,取得更加有效的治疗效果。 2.1.2 磁场敏感型凝胶 Su等[16]在磁场作用下使氧化铁磁性纳米颗粒标记的软骨细胞均匀分布于聚乳酸-羟基乙酸共聚物与壳聚糖-Ⅱ型胶原双层支架,细胞支架染色证实支架材料逐渐转变为软骨组织,软骨外基质填充支架空隙中,RT-qPCR也证实了特异基因的表达。该水凝胶运用磁场达到种子细胞的移动性,使负载的细胞均匀覆盖损伤部位,仿生双层结构上层的细胞在适宜环境下短时间内促进软骨细胞增殖,为分泌的细胞外基质提供黏附点,同时为再生血管化提供条件,下层聚乳酸-羟基乙酸共聚物层则成为软骨细胞迁移的过渡区,为整个支架结构的移植提供机械稳定性。但目前磁铁矿纳米粒子的体内相容性尚不明确,还需进一步研究。 2.2 种子细胞-壳聚糖体系水凝胶 种子细胞作为组织工程重要组成部分之一,同种软骨细胞获取简单,且同种自体具有低免疫原性风险。Chen等[17-18]将兔自体软骨细胞封装于乙二醇壳聚糖水凝胶支架内,用于修复兔膝关节软骨缺损,发现体外维持软骨细胞形态并使细胞增殖超过21 d,植入体内后,水凝胶为携带的软骨细胞分泌细胞外基质提供附着,缺损部位Ⅱ型胶原蛋白呈高表达,软骨缺损明显改善。但从自体非负重区可取软骨有限,体外扩增自体软骨移植在短期内需进行2次手术治疗,对患者的经济及身心压力产生了较大考验,极大限制了该手术的临床应用[19]。Man等[20]运用去矿化骨基质-壳聚糖水凝胶支架搭载同种异体软骨细胞体内修复兔软骨损伤,术后24周,支架组未见明显炎症反应,支架具备良好组织相容性,组织学评分明显优于对照组,MRI显示支架组软骨缺损被成功填充,去矿化骨基质给予水凝胶稳定的支架结构,壳聚糖为搭载种子细胞提供了良好的黏附环境,有效修复了软骨缺损。但同种异体细胞可能存在免疫排斥反应和疾病传播风险[21]。无论自体或异体软骨细胞负载,软骨细胞在体外扩增培养细胞过程中存在去分化现象,可能失去原有细胞的形态及功能[8]。 自体和异体软骨细胞移植受到来源及运用的限制,间充质干细胞、脂肪源性干细胞逐渐成为再生医学中使用最广泛的干细胞[22-23],因为它们具有丰富的细胞来源和低免疫原性,畸胎瘤风险极小,在三维环境下接受特定物理刺激或化学诱导具有增殖、分化成软骨细胞的能力[24-26]。Lu等[27]将间充质干细胞搭载于壳聚糖膜并诱导其成软骨分化,在染色鉴定下证实中等分子质量(190-310 kDa)的壳聚糖薄膜更适于诱导间充质干细胞向软骨分化,分泌软骨形成蛋白及软骨内标记呈现高表达。此外,Zhu等[28]模拟关节运动剪切力,在壳聚糖/明胶复合水凝胶支架中种植人脂肪源性干细胞,运用旋转壁容器生物反应器干预,动态与静态培养构建工程软骨仿生材料,结果证实在诱导培养基动态培养下,脂肪源性干细胞呈现软骨细胞外基质广泛表达,有效促进了脂肪源性干细胞的增殖及成软骨分化。 随着体外研究成果的进一步推进,有研究在兔软骨缺损体内植入负载骨髓间充质干细胞的聚乙烯醇/壳聚糖复合水凝胶,验证得到复合支架材料体外细胞相容性良好,体内对兔软骨缺损有明显的修复效果,但可能由于体内观察时间较短和植入材料过少的缘故,软骨缺损未完全修复。林涛等[29]运用复合兔脂肪源性干细胞及肋软骨细胞的壳聚糖水凝胶修复兔膝关节软骨缺损,发现双细胞支架组在12周时达到完全修复且缺损边界模糊,再生组织富含Ⅱ型胶原蛋白,达到透明软骨修复的效果。但干细胞存在多向分化能力,存在诱发体内肿瘤发病的潜在风险,如何定向诱导干细胞成软骨及成骨分化还需进一步的深入研究。 2.3 生长因子-壳聚糖体系水凝胶 干细胞是软骨再生的起点,但必须将干细胞诱导分化形成软骨组织,可最终达到形成透明软骨。大量实验证明,转化生长因子β、胰岛素样生长因子、骨形态发生蛋白和地塞米松等是最广泛用于刺激干细胞软骨形成、成骨分化的活性因子与激素[30-33]。其中生长转化因子β是一种具有成熟二聚体, 的25 kDa多肽[34]。大量体内外实验证明,活化的转化生长因子β信号通路是胚胎发生软骨形成的驱动因素,不同来源干细胞在与活化转化生长因子β共培养下可形成软骨组织[31-35]。Merlin Rajesh Lal等[36]将壳聚糖的细胞黏附性和琼脂糖优良的机械稳定性相结合,采用冷冻干燥合成了壳聚糖-琼脂糖凝胶,封装人脐带华通胶间充质干细胞后,在双生长因子骨形态发生蛋白2、转化生长因子β3诱导下成功向软骨分化,壳聚糖-琼脂糖凝胶的低免疫原性可更好地支持同种异体人脐带华通胶间充质干细胞分化,双因子支架对人脐带华通胶间充质干细胞的诱导分化较单纯支架更为显著,软骨标志物COL2A1、ACAN、SOX6和SOX9均呈高表达。但无论骨形态发生蛋白2或转化生长因子β3,体外制备复杂、昂贵且体外保存稳定性较差,使其临床运用受到一定限制。 富血小板血浆来源于自体血小板浓缩物,分离与提取快捷方便,而血小板在凝血酶或CaCl2作用下活化形成α纯富血小板血浆,后者含有多种生长因子(转化生长因子β、胰岛素样生长因子或血管内皮生长因子)[37-39]。Sancho-Tello等[40]在稳定多孔壳聚糖上结合富血小板血浆,结果显示与空载支架对比,富血小板血浆负载壳聚糖支架在多重生长因子及稳定纤维网络影响下可促使软骨细胞Ⅱ型胶原蛋白高表达,且电镜扫描观察到富血小板血浆诱导分化软骨细胞与正常软骨细胞形态结构相一致,为软骨修复提供了新的思考方向。 2.4 基因-壳聚糖体系水凝胶 生长因子对加速、诱导细胞增殖有不可或缺的作用,研究者逐渐将目光转移到转染生长因子,提高生长因子生物效力,从基因分子机制层面来增强软骨修复效率的研究中。赵荣兰等[41-42]研究发现,壳聚糖/胰岛素样生长因子基因复合物在体外能促进间充质干细胞的增殖速度并向软骨分化。随后的兔体内实验中,运用荧光定量PCR对修复部位基因进行定量检测,发现壳聚糖/胰岛素样生长因子基因干预组Ⅱ型胶原、聚集蛋白聚糖基因均呈上调趋势,组织修复效果明显高于对照组。此外,该课题组还构建了磷酸化短肽偶联壳聚糖基因载体,负载胰岛素样生长因子1和白细胞介素1受体拮抗剂双基因,用于修复兔软骨损伤,验证得到双基因在软骨修复过程中存在协同作用,聚集蛋白聚糖无论基因或蛋白层面均呈现高表达,组织学染色中发现软骨基质的分解代谢被抑制,合成代谢被增强[43-44]。 2.5 基质-壳聚糖体系水凝胶 种子细胞、生长因子的负载存在一定潜在风险,广大学者开始探索运用软骨细胞外基质类似物制作脱细胞支架,从而规避疾病传播、诱发潜在畸胎瘤等临床风险,同时为原有组织增殖、黏附提供骨架材料[45]。Sivandzade等[46]在电喷雾技术下将人软骨组织脱细胞细胞外基质包封于壳聚糖水凝胶中,制备缓释微球,1%细胞外基质:2%壳聚糖的缓释微球在孔径、密度、孔隙率和机械性质方面表现出独特优势,此外在体外动态与静态共培养原代人软骨细胞对比中发现,动态培养下1%细胞外基质缓释微球具备更佳的细胞迁移、增殖等能力,能达到最大的细胞附着,为后期软骨修复支架材料提供参考。 2.6 3D打印仿生壳聚糖体系水凝胶 随着对软骨组织结构了解的不断深入,3D打印技术突飞猛进,模拟软骨结构修复支架材料的理念进入学者们的视野[7,47]。Reed等[48]应用3D打印技术模拟软骨及软骨下骨结构,将天然关节软骨细胞外基质糖胺聚糖类似多糖与壳聚糖-藻酸盐定向冷冻制备为多微孔无细胞支架,该支架实现分层模拟关节软骨分层体系,有助于摄取内源性骨髓、再生细胞迁移分布和新生软骨血管化。Wang等[49]利用壳聚糖改性多糖-羟丁基壳聚糖,通过控制其表面羟丁基取代度调控多糖-羟丁基壳聚糖溶液临界温度溶胶-凝胶状态的转变,于10%NaCl溶液中,支架在73.2 kPa- 40 MPa下表现出优异的可调弹性和弹力回缩性,具有良好的生物降解性和细胞相容性。该水凝胶在3D打印技术支持下可制得具有强机械性能和精细结构的25层支架材料,有望应用于软骨组织的修复。"
| [1] Zhu J, Marchant RE.Design properties of hydrogel tissue-engineering scaffolds.Expert Rev Med Devices. 2011; 8(5):607-626.[2] Oryan A, Sahvieh S.Effectiveness of chitosan scaffold in skin, bone and cartilage healing.Int J Biol Macromol.2017;104(Pt A): 1003-1011.[3] 徐敬,赵建宁,徐海栋,等.壳聚糖及其衍生物在软骨组织工程中的应用[J].中国组织工程研究, 2015,19(25):4081-4085.[4] 陈道玉,张中民,姜丽丽.壳聚糖支架材料在组织工程中的应用与未来[J].中国组织工程研究,2017, 21(30):4893-900.[5] Ali A, Ahmed S.A review on chitosan and its nanocomposites in drug delivery.Int J Biol Macromol. 2018;109:273-286.[6] Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R,et al. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine.Biomed Res Int. 2015; 2015:821279.[7] Radhakrishnan J, Subramanian A, Krishnan UM,et al. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules.2017;18(1):1-26.[8] Nukavarapu SP,Dorcemus DL.Osteochondral tissue engineering: current strategies and challenges.Biotechnol Adv. 2013;31(5):706-721.[9] Ahmed S, Annu, Ali A, et al. A review on chitosan centred scaffolds and their applications in tissue engineering.Int J Biol Macromol. 2018;116:849-862.[10] 傅月荷,吕青.生物来源水凝胶在组织工程中的应用与进展[J].中国修复重建外科杂志,2014,28(8):1030-1036.[11] Chenite A, Chaput C, Wang D, et al.Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials.2000;21(21):2155-2161.[12] 康肸,邓爱鹏,杨树林.壳聚糖基温敏水凝胶的研究进展[J].中国生物工程杂志,2018,38(5):79-84.[13] Wu J, Liu J, Shi Y,et al. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/ hydroxyapatite/glycerophosphate hydrogels.J Mech Behav Biomed Mater. 2016;64:161-172.[14] Song K, Li L, Yan X,et al. Characterization of human adipose tissue-derived stem cells in vitro culture and in vivo differentiation in a temperature-sensitive chitosan/β- glycerophosphate/collagen hybrid hydrogel.Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):231-240.[15] Deng A, Kang X, Zhang J, et al. Enhanced gelation of chitosan/β-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated.Mater Sci Eng C Mater Biol Appl. 2017;78: 1147-1154.[16] Su JY, Chen SH, Chen YP, et al. Evaluation of Magnetic Nanoparticle-Labeled Chondrocytes Cultivated on a Type II Collagen-Chitosan/Poly(Lactic-co-Glycolic) Acid Biphasic Scaffold.Int J Mol Sci. 2017;18(1). pii: E87. doi: 10.3390/ijms 18010087. [17] Chen Z, Zhao M, Liu K,et al. Novel chitosan hydrogel formed by ethylene glycol chitosan, 1,6-diisocyanatohexan and polyethylene glycol-400 for tissue engineering scaffold: in vitro and in vivo evaluation.J Mater Sci Mater Med. 2014; 25(8): 1903-1913.[18] Zhao M, Chen Z, Liu K, et al. Repair of articular cartilage defects in rabbits through tissue-engineered cartilage constructed with chitosan hydrogel and chondrocytes.J Zhejiang Univ Sci B. 2015;16(11):914-923.[19] Oussedik S, Tsitskaris K, Parker D.Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy. 2015;31(4):732-744.[20] Man Z, Hu X, Liu Z,et al.Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157-167.[21] Song K, Li L, Yan X, et al. Fabrication and development of artificial osteochondral constructs based on cancellous bone/hydrogel hybrid scaffold.J Mater Sci Mater Med. 2016; 27(6):114. [22] 刘相杰,宋科官.生物支架材料及间充质干细胞在骨组织工程中的研究与应用[J].中国组织工程研究, 2018,22(10):1618-24.[23] Dai R, Wang Z, Samanipour R,et al. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications.Stem Cells Int.2016;2016:6737345.[24] Tamaddon M, Burrows M, Ferreira SA,et al. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro.Sci Rep. 2017;7:43519.[25] Yang J,Zhang YS,Yue K,et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering.Acta Biomater. 2017;57:1-25.[26] Huang H, Zhang X, Hu X, et al. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering.Biomed Mater. 2014;9(3):035008. [27] Lu TJ,Chiu FY,Chiu HY,et al. Chondrogenic Differentiation of Mesenchymal Stem Cells in Three-Dimensional Chitosan Film Culture.Cell Transplant. 2017;26(3):417-427.[28] Zhu Y,Song K,Jiang S,et al. Numerical Simulation of Mass Transfer and Three-Dimensional Fabrication of Tissue-Engineered Cartilages Based on Chitosan/Gelatin Hybrid Hydrogel Scaffold in a Rotating Bioreactor.Appl Biochem Biotechnol. 2017;181(1):250-266.[29] 林涛.壳聚糖水凝胶复合脂肪间充质干细胞修复兔关节软骨缺损[J].中华创伤杂志,2016,32(4):357-362. [30] Madry H, Rey-Rico A, Venkatesan JK, et al. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering.Tissue Eng Part B Rev. 2014;20(2):106-125.[31] Yang YH, Barabino GA.Differential morphology and homogeneity of tissue-engineered cartilage in hydrodynamic cultivation with transient exposure to insulin-like growth factor-1 and transforming growth factor-β1.Tissue Eng Part A. 2013;19(21-22):2349-2360.[32] Madry H, Kaul G, Zurakowski D, et al.Cartilage constructs engineered from chondrocytes overexpressing IGF-I improve the repair of osteochondral defects in a rabbit model.Eur Cell Mater. 2013;25:229-247.[33] Kessler MW,Grande DA.Tissue engineering and cartilage. Organogenesis.2008;4(1):28-32.[34] Sporn MB, Roberts AB, Wakefield LM, et al.Transforming growth factor-beta: biological function and chemical structure. Science.1986;233(4763):532-453.[35] Ahsan SM, Thomas M, Reddy KK, et al. Chitosan as biomaterial in drug delivery and tissue engineering.Int J Biol Macromol.2018;110:97-109.[36] Merlin Rajesh Lal LP, Suraishkumar GK, Nair PD. Chitosan-agarose scaffolds supports chondrogenesis of Human Wharton's Jelly mesenchymal stem cells.J Biomed Mater Res A.2017;105(7):1845-1855.[37] Shen J, Gao Q, Zhang Y,et al. Autologous platelet?rich plasma promotes proliferation and chondrogenic differentiation of adipose?derived stem cells.Mol Med Rep. 2015;11(2):1298-1303.[38] Krüger JP,Ketzmar AK,Endres M,et al.Human platelet-rich plasma induces chondrogenic differentiation of subchondral progenitor cells in polyglycolic acid-hyaluronan scaffolds.J Biomed Mater Res B Appl Biomater.2014;102(4):681-692.[39] Sadeghi-Ataabadi M,Mostafavi-Pour Z,Vojdani Z,et al. Fabrication and characterization of platelet-rich plasma scaffolds for tissue engineering applications.Mater Sci Eng C Mater Biol Appl. 2017;71:372-380.[40] Sancho-Tello M, Martorell S, Mata Roig M, et al. Human platelet-rich plasma improves the nesting and differentiation of human chondrocytes cultured in stabilized porous chitosan scaffolds.J Tissue Eng. 2017;8:2041731417697545.[41] 赵荣兰,左爱军,梁东春,等.CS/igf-1基因复合物促骨髓基质细胞增殖及分化的研究[J].天津医药,2008,36 (1):35-37.[42] 赵荣兰,任玉平,孙蓓,等.壳聚糖转染胰岛素样生长因子基因促进兔关节软骨损伤修复的实验研究[J].中国修复重建外科杂志, 2010,24(11):1372-1375.[43] 赵荣兰,彭效祥,楚海荣,等.可磷酸化短肽偶联壳聚糖介导兔关节软骨损伤修复的基因治疗[J].中国修复重建外科杂志, 2014, 28(11):1346-52.[44] 赵荣兰,彭效祥,宋伟,等.可磷酸化短肽偶联壳聚糖介导IGF-1和IL-1RA双基因联合治疗兔关节软骨损伤[J].中国生物化学与分子生物学报,2015,31(2):175-181.[45] Kiyotake EA, Beck EC, Detamore MS.Cartilage extracellular matrix as a biomaterial for cartilage regeneration.Ann N Y Acad Sci. 2016;1383(1):139-159.[46] Sivandzade F, Mashayekhan S.Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan.J Biomater Sci Polym Ed.2018;29(6):683-700.[47] Medeiros Borsagli FGL, Carvalho IC, Mansur HS. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications.Int J Biol Macromol. 2018;114:270-282.[48] Reed S, Lau G, Delattre B, et al. Macro- and micro-designed chitosan-alginate scaffold architecture by three-dimensional printing and directional freezing. Biofabrication. 2016;8(1): 015003.[49] Wang X, Wei C, Cao B, et al. Fabrication of Multiple-Layered Hydrogel Scaffolds with Elaborate Structure and Good Mechanical Properties via 3D Printing and Ionic Reinforcement.ACS Appl Mater Interfaces. 2018;10(21): 18338-18350. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||