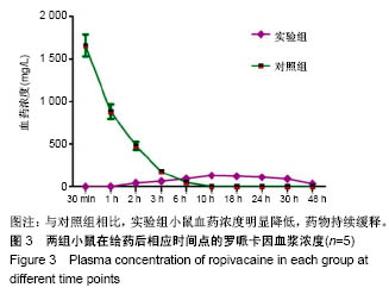
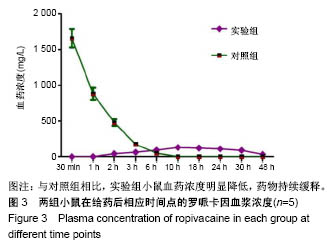
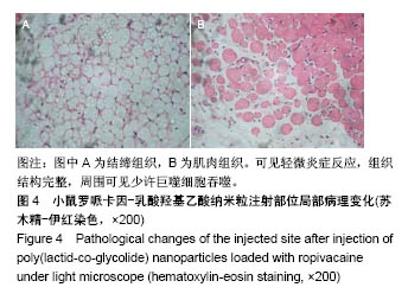
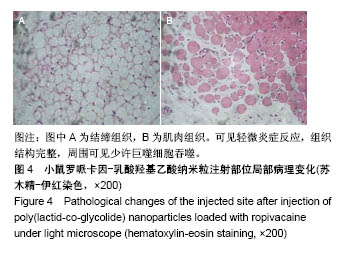
| [1] Foley PL, Ulery BD, Kan HM, et al. A chitosan thermogel for delivery of ropivacaine in regional musculoskeletal anesthesia. Biomaterials. 2013;34(10):2539-2546. [2] Zhang W, Ning C, Xu W, et al. Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere. Theranostics. 2018;8(12):3331-3347. [3] Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline From the American Pain Society. the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. [4] Grant GJ, Piskoun B, Bansinath M. Analgesic duration and kinetics of liposomal bupivacaine after subcutaneous injection in mice. Clin Exp Pharmacol Physiol. 2003;30(12):966-968. [5] Joshi G, Gandhi K, Shah N, et al. Peripheral nerve blocks in the management of postoperative pain: Challenges and opportunities. J Clin Anesth. 2016;35(12):524-529. [6] Zorzetto L, Brambilla P, Marcello E, et al. From micro- to nanostructured implantable device for local anesthetic delivery. Int J Nanomed. 2016;11:2695-709. [7] Masters DB, Berde CB, Dutta S, et al. Sustained local anesthetic release from bioerodible polymer matrices: a potential method for prolonged regional anesthesia. Pharm. Res. 1993;10(10):1527-1532. [8] Masters DB, Berde CB, Dutta SK, et al. Prolonged regional nerve blockade by controlled-rease of local anesthetics from a biodegradable polymer matrix. Anesthesiology. 1993; 79(2): 340-346. [9] Morgalla M, Fortunato M, Azam A, et al. High-resolution three-dimensional computed tomography for assessing complications related to intrathecal drug delivery. Pain Physician. 2016;19(5):E775-780. [10] Masters DB, Domb AJ. Liposphere local anesthetic timed-release for perineural site application. Pharm. Res. 1998;15(7):1038-1045. [11] Santamaria CM, Woodruff A, Yang R, et al. Drug delivery systems for prolonged duration local anesthesia. Mater Today. 2017;20(1):22-31. [12] Burbridge M, Jaffe RA. Exparel (R): A new local anesthetic with special safety concerns. Anesth Analg. 2015;121(4): 1113-1114. [13] de Paula E, Cereda CM, Fraceto LF, et al. Micro and nanosystems for delivering local anesthetics. Expert Opin. Drug Deliv. 2012;9 (12):1505-1524. [14] Mulroy MF, Larkin KL, Batra MS, et al. Femoral nerve block with 0. 25% or 0. 5% bupivacaine improves postoperative analgesia following outpatient arthroscopic anterior cruciate ligament repair. Reg. Anesth. Pain Med. 2001;26 (1):24-29. [15] Davidson EM, Barenholz Y, Cohen R, et al. High-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humans. Anesth Analg. 2010;110 (4):1018-1023. [16] Ni Q, Chen W, Tong L, et al. Preparation of novel biodegradable ropivacaine microspheres and evaluation of their efficacy in sciatic nerve block in mice. Drug Des Dev Ther. 2016;10:2499. [17] Sivakumaran D, Maitland D, Oszustowicz T, et al. Tuning drug release from smart microgel–Gel composites via cross-linking. J Colloid Interface Sci. 2013;392:422-430. [18] Andhariya JV, Shen J, Choi S, et al. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J Controlled Release. 2017;255:27-35. [19] Belz JE, Kumar R, Baldwin P, et al. Sustained release talazoparib implants for localized treatment of BRCA1-deficient breast cancer. Theranostics. 2017;7(17): 4340-4349. [20] Hu Y, Hoerle R, Ehrich M, et al. Engineering the lipid layer of lipid-PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015;85(30): 30125-30132. [21] Mandal B, Bhattacharjee H, Mittal N, et al. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9(4):474-491. [22] Wang T, Hurwitz O, Shimada SG, et al. Anti-nociceptive effects of bupivacaine-encapsulated PLGA nanoparticles applied to the compressed dorsal root ganglion in mice. Neurosci Lett. 2018;6 (68):154-158[23] Liu J, Lv X. The pharmacokinetics and pharmacodynamics of lidocaine-loaded biodegradable poly(lactic-co-glycolic acid) microspheres. Int J Mol Sci. 2014;15(10):17469-17477. [24] Ohri R, Blaskovich P, Wang JCF, et al. Prolonged nerve block by microencapsulated bupivacaine prevents acute postoperative pain in rats. Reg Anesth Pain Med. 2012;37(6): 607-615. [25] Cheow WS, Hadinoto K. Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf B Biointerfaces. 2011;85 (2):214-220. [26] 毕小宝,陈仲清,黄乐松,等.罗哌卡因乳酸羟基乙酸共聚物微球的制备及体外释药研究[J].中国药房,2008,19(13):0430-438.[27] 田洪居,陈仲清,王照飞,等.罗哌卡因乳酸羟基乙酸共聚物微球在小鼠的坐骨神经阻滞时效及生物相容性[J].临床麻醉学杂志, 2010,26(2):148-150。[28] 方天,田迎,王建东,等.溶液中稳定分散的介孔二氧化硅纳米球的制备及其生物毒性研究[J].医学研究生学报, 2013,26(10): 1014-1019.[29] Ma P, Li T, Xing H, et al. Local anesthetic effects of bupivacaine loaded lipid-polymer hybrid nanoparticles: In vitro and in vivo evaluation. Biomed Pharmacother. 2017;8(9): 689-695 [30] Kopacz DJ, Bernards CM, Allen HW, et al. A model to evaluate the pharmacokinetic and pharmacodynamic variables of extended-release products using in vivo tissue microdialysis in humans: bupivacaine-loaded microcapsules. Anesth Analg. 2003;97(1):124-131. |