Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (30): 4889-4896.doi: 10.3969/j.issn.2095-4344.1424
Previous Articles Next Articles
Molecular biological mechanism of intervertebral disc degeneration and the advantages and prospects of regenerative therapy
- 1Graduate School of Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; 2Department of Spinal Surgery, the People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2019-05-25Online:2019-10-28Published:2019-10-28 -
Contact:Jianxun, Master, Chief physician, Master’s supervisor, Department of Spinal Surgery, the People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Yu Chengqiang, Master candidate, Graduate School of Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; Department of Spinal Surgery, the People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 2016GXNSFAA380058 (to WJX)
CLC Number:
Cite this article
Yu Chengqiang, Zhang Yu, Xie Chengxin, Ou Yufu, Wei Jianxun. Molecular biological mechanism of intervertebral disc degeneration and the advantages and prospects of regenerative therapy[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(30): 4889-4896.
share this article
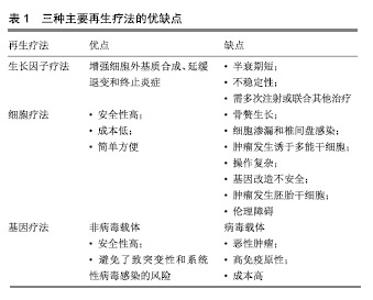
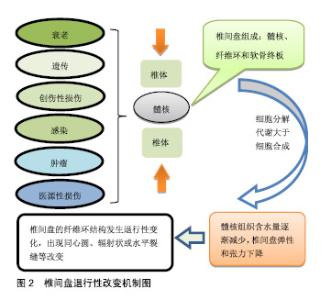
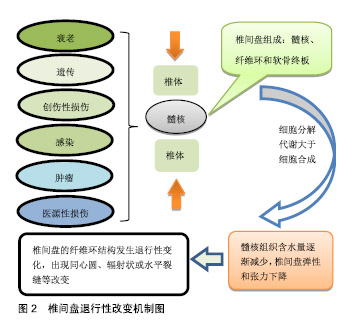
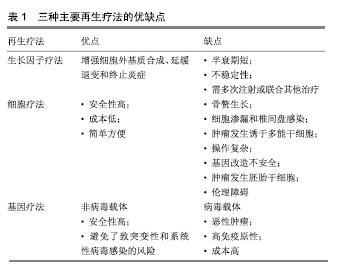
然而,当前治疗椎间盘退变所引起的临床症状,主要是保守治疗和手术治疗,当药物保守治疗未能缓解神经压迫产生的疼痛时,外科干预就会成为首选的治疗方式[17]。迄今为止,这种导致背部和颈部疼痛的疾病,除了手术之外几乎没有可用的其他治疗方法[18],并且这种治疗只侧重于症状性疼痛的缓解,而不是针对椎间盘退化过程的病理生理学进行治疗[19],因此,保守治疗和外科介入治疗效率有限,且多数病例缺乏相对持久的效 果[20-21]。另外手术治疗也存在着高侵袭性和高复发性的风险,以及导致椎间盘机械性能丧失和邻近脊柱节段退变的缺点[22]。 随着医学分子生物学的发展和椎间盘退变的相关分子的深入研究,研究者对椎间盘退变的治疗已转向再生医学领域,他们的研究热点主要集中在维持椎间盘内环境的稳定和逆转退化的过程。更有研究人员在探索椎间盘再生的新疗法中,将其归纳为生长因子治疗、细胞治疗和基因治疗3类[22-23],见表1。 2.2 椎间盘退变的再生疗法 2.2.1 生长因子疗法 生长因子被认为是一类具有增加椎间盘功能细胞数目或能加强椎间盘内源性细胞功能的多肽类物质,促进椎间盘细胞的再生或恢复。生长因子作用于椎间盘细胞表面的特定受体,影响细胞外基质的增殖、分化和合成。特定生长因子如骨形态发生蛋白和转化生长因子β(TGF-β)的成员可以刺激骨和软骨的形成[24]。研究表明,转化生长因子(TGF)、表皮生长因子(EGF)、碱性成纤维细胞生长因子(bFGF)、骨形态发生蛋白(BMP-2,7)、生长和分化因子5(GDF-5)和胰岛素样生长因子1(IGF-1)可诱导细胞外基质成分的合 成[25-27]。Yang等[28]在研究中表示,白细胞介素1β通过刺激多种炎症递质的分泌,而具有强烈的促炎活性,且在退行性椎间盘组织和细胞中有着高表达,已经显示其参与椎间盘退变期间的多种病理过程,包括炎症反应、基质破坏、血管生成和神经支配、细胞凋亡、氧化应激和细胞衰老。通过对白细胞介素1β的抑制将有利于促进细胞外基质的修复和再生。另外,在一项回顾性研究中,Li等[29]总结出骨形态发生蛋白1在延迟椎间盘退变和再生椎间盘方面十分有效,为生物学治疗退化椎间盘提供了可行性选择。 生长因子治疗是生物学策略之一,具有广阔的前景。富含血小板血浆作为一种有前途的生物活性物质,在调节组织微环境、改善细胞功能、促进受损组织的再生中发挥重要作用,被认为是椎间盘修复的理想生长因子[30]。许多体外和体内研究的结果证实,生长因子和富含血小板血浆具有促进椎间盘退变修复和再生的功效[31-33],加大对椎间盘退变的分子生物学的研究将有助于推进生长因子治疗椎间盘退变的研究。 生长因子能增强细胞外基质合成、延缓退变和终止炎症的作用。Feng等[34]表示生长和分化因子5是用于椎间盘退变再生治疗的首选因子,是迄今为止发现与椎间盘退变相关的少数生长因子之一,能维持椎间盘的结构和功能。Tran等[35]相关研究表示结缔组织生长因子(CCN2/CTGF)在细胞外基质的合成中起重要作用,特别是在骨骼组织如软骨、骨和椎间盘中,转化生长因子β对结缔组织生长因子进行调节在细胞外基质的合成中具有重要的意义。Kennon等[26]在研究原型生长因子和生长因子组合的作用时,发现它们具有恢复椎间盘结构的能力,并且表示使用生长因子可以作为恢复椎间盘细胞的潜在的治疗方法。Shen等[36]在研究椎间盘退变时,通过将生长和分化因子5与对照组进行比较,发现肿瘤坏死因子α、白细胞介素1和前列腺素E2蛋白表达水平以及培养液中一氧化氮浓度上调,而生长和分化因子5的表达显著的抑制其上调。生长和分化因子5诱导的人脂肪来源干细胞可以分化成髓核样表型,这种基于干细胞的疗法被认为是有希望的治疗,但此方法在缓解椎间盘退变的程中的功效受限于生长因子的短寿命。Zhu等[37]使用由肝素和合成聚阳离子poly(ethylene argininylaspartate diglyceride,PEAD)组成的独特生长因子递送载体来维持生长和分化因子5的释放。结果显示PEAD:肝素递送系统持续释放生长和分化因子5促进人脂肪来源干细胞在体外分化为髓核样表型。 生长因子在椎间盘退变的再生治疗中拥有很大的研究价值。然而,因为椎间盘退变是属于慢性疾病,且生长因子的半衰期又短,需要多次注射生长因子或组合多种生长因子才能达到治疗的目的,另外生长因子的不稳定性又限制了将它们直接注射至椎间盘的可能。 2.2.2 细胞治疗法 椎间盘退变的主要原因通常是髓核细胞数量的减少,细胞疗法的新进展为椎间盘退变的治疗提供新颖且有效的策略。它是通过从移植细胞的旁分泌信号传导来刺激椎间盘细胞,或通过积极参与细胞外基质的产生和体内平衡来实现[38],其主要的目的是补充和替换坏死或凋亡的细胞,使椎间盘细胞退变的程度控制到最小[39-40]。 间充质干细胞和幼年髓核细胞能够重新激活退化的髓核细胞[41-42]。由于椎间盘中缺乏活跃的细胞群和有限的细胞募集潜力,许多研究都旨在将细胞群引入椎间盘,以此使被引入的细胞可以原位结合,并产生新的基质或通过营养因子刺激邻近细胞[34]。髓核细胞的重新插入可以进一步减缓椎间盘的退变,在研究髓核细胞移植中,Mochida等[43]通过使用动物模型和人类细胞的比较,发现髓核细胞移植在退化腰椎间盘中的安全性和有效性更高,并在3年的随访期间没有发现不良反应,也没有病例报告相关的腰痛。同时,Xia等[44]认为源自胚胎脊索的髓核祖细胞不仅可以在椎间盘退变的严重缺氧环境中存活,还可以有效地分化成髓核样细胞,并且预测使用多能干细胞和髓核祖细胞来修复退变椎间盘细胞的新疗法。 当前的临床研究中最常用的细胞类型主要是间充质干细胞,骨髓来源的间充质干细胞是一种具有再生软骨、骨和纤维组织的能力的干细胞。Yoshikawa等[45]从2例腰椎管狭窄患者的髂骨中收集骨髓液以培养骨髓间充质干细胞,并将其移植到退化的椎间盘中,2年后通过X射线片和计算机断层扫描显示2名患者的真空现象都有所改善。同种异体间充质干细胞作为治疗退行性椎间盘疾病的有效替代方案[46],其在应用上不仅比自体间充质间质干细胞简单方便,而且在缓解疼痛的同时,还改善了退变椎间盘的功能。在24例被诊断为腰椎间盘退变的患者中,Noriega等[47]在局麻下通过向椎间盘内注射同种异体骨髓间充质干细胞,与对照组相比,实验组的疼痛和残疾评分显著改善。间充质间质干细胞在椎间盘退变的治疗已显示出可行性、安全性和临床疗效适应性。 细胞移植是一种安全有效的方法,为椎间盘细胞的再生治疗提供了一种低风险、低成本的策略,具有潜在的长期治疗价值,但是细胞疗法存在着细胞渗漏和骨赘形成的不良反应,在保留、存活和维持活性方面也有一定的困难。为了克服这些局限性,基于组织工程的细胞疗法变得更为突出。组织工程是利用种子细胞、生物支架和生物活性物质联合使用,支架被设计成可注射的形式用于微创治疗。细胞治疗面临着诸多挑战和障碍,其所使用的平台将不得不模仿高度结构化的结构,并经历拉伸和剪切应力[48]。因此,细胞治疗可能不利于晚期椎间盘退变。 2.2.3 基因治疗法 遗传是导致椎间盘退变最普遍的危险因素,通过研究遗传基因与椎间盘退变的关系,将为延迟或恢复退变椎间盘细胞的提够更多的选择,Mayer等[49]发现有20种基因多态性编码在椎间盘退变机制中起作用,其中最明显基因包括以下5种,维生素D受体、聚集蛋白聚糖、基质金属蛋白酶3、IX型胶原蛋白及无孢蛋白。另外,在退行性椎间盘组织中miRNA也被发现存在着一定的表达,Wang等[10]表示miRNA的失调可以加快椎间盘退变的进程,它不仅能引起髓核和细胞外基质发生炎症反应,还能引起软骨终板发生变性,导致细胞凋亡。对此,有研究人员表示基因疗法具有减弱或逆转椎间盘内的退行性过程[50],在椎间盘退变的再生治疗中,基因治疗的基础是诱导椎间盘内基因表达的变化,这些基因通过载体进行体内基因治疗,或者直接注射到细胞中,或者通过使用靶细胞进行体外基因治疗,在体外将这些靶细胞去除、培养、基因改变,然后再重新植入相关的部位[51]。基因治疗所需要的载体可分为病毒载体和非病毒载体两类,其中病毒载体因具有高效的复制增殖的能力而被广泛应,传统的病毒载体包括逆转录病毒、慢病毒、腺病毒、腺相关病毒和杆状病毒[22],而非病毒载体因没有达到病毒载体的功效,目前仍处于研发之中。 病毒载体转导的基因治疗:1997年,Wehling等[52]首次使用逆转录病毒成功地将-半乳糖苷酶基因从牛软骨终板转移到软骨细胞中,取得了预期效果。通过这种方法避免了传统药物和手术治疗椎间盘退变带来的不利影响,为椎间盘退变的修复或逆转开启了新时代。慢病毒被认为是一种用于椎间盘细胞的有效且稳定的转导载体,它具有作为治疗人类退行性椎间盘疾病的基因治疗工具的潜力,在研究体外和体内慢病毒载体对椎间盘细胞的转导效率和可持续性中,Zhang等[53]从28例患者的椎间盘组织中分离出核髓和纤维环细胞,使用重组慢病毒进行转导,结果表明不同剂量的慢病毒转导的椎间盘细胞与对照组细胞相比具有同样良好的活力。Lei等[54]将携带骨形态发生蛋白2和分化抑制因子1(Id1)基因的慢病毒(Lv)载体直接注射到新西兰白兔髓核中,实验结果表明Lv-BMP-2和Lv-Id1的组合应用可以延迟椎间盘退变的进一步发展。Liu等[3]通过15只新西兰大白兔建立的腰椎间盘退变模型中,使用慢病毒载体对其进行干预,最后MRI结果显示,实验组的椎间盘退变得到了改善,最终结果表明基因治疗在椎间盘退变的再生治疗中的具有一定的可行性。有研究者表示细胞凋亡、分解代谢活性增加和合成代谢活性降低是椎间盘退变的机制。同时,survivin蛋白、转化生长因子β3(TGFB3)和金属蛋白酶组织抑制因子1(TIMP1)可分别影响上述过程。Yue等[55]将携带慢病毒的survivin-TGFB3-TIMP1注射到穿刺的兔椎间盘中有助于延迟退行性椎间盘的变化。虽然动物模型的数据应该谨慎推断人类病情,但这项研究显示基因疗法有望减缓椎间盘退变。 腺病毒是基因传递的有效载体,被广泛应用于基因治疗,并且具有在体外和体内模型中有效感染非分裂细胞并帮助高水平瞬时基因表达的优点。在研究椎间盘退变的候选基因中,Luo等[56]使用AdEasy-1腺病毒载体系统分离,培养并用腺病毒介导的GDF-5(Ad-GDF-5)感染人退化椎间盘的髓核细胞,最终只有Ad-GDF-5组中的细胞促进了细胞外基质的产生和髓核细胞的增殖。对于退变纤维环的基因治疗,大多数研究使用腺病毒和腺相关病毒作为载体[57]。为研究腺病毒转导的骨形态发生蛋白4对修复椎间盘退变细胞的影响,Cao等[58]从患者的退行性腰椎间盘中吸取退行性腰椎间盘细胞进行对照研究,随后发现实验组蛋白多糖、Ⅱ型胶原和Sox9的mRNA表达均显著高于对照组,可见腺病毒人骨形态发生蛋白4(Ad-hBMP-4)可以高效转染人退行性腰椎间盘细胞,促进Ⅱ型胶原蛋白、蛋白多糖和Sox9表达。此外,Ren等[59]在兔椎间盘退变模型中测试了腺相关病毒介导的Sox-9和OP-1基因共转染的功效,并且MRI信号显示,椎间盘高度,基因表达和基质分子显着增加。 杆状病毒作为一种新的基因治疗转递载体具有较高的安全性,不会造成动物细胞的功能损害[60],且不会在细胞内的增殖与和扩散[61],在作为椎间盘退变基因治疗的载体中,具有广大的应用前景。 基因治疗椎间盘退变已显示出巨大应用前景,然而,在治疗过程中所产生的问题仍需高度注意。使用逆转录病毒载体的最大问题在于引起的潜在恶性肿瘤。腺病毒载体的缺点是高度免疫原性,导致对外来转基因编码蛋白的主要免疫应答,这有可能限制该技术的有效性。用于基因转位的病毒载体除了对患者仍然未知的风险外,在制备过程中也承担着重大费用。因此,研究并开发出低风险、低成本的非病毒基因载体已迫在眉睫。 非病毒载体转导的基因治疗:非病毒基因载体安全性高,避免了突变和病毒感染的风险,具有解决病毒性基因治疗带来的风险[62-63]。这一载体包括脂质体,DNA-配体复合物和基因枪技术[22],将是研究椎间盘退变再生疗法的主要领域。当前,开发的非病毒性传播剂之一是通过超声穿孔传送的微泡,这种技术是使用微泡携带质粒DNA,通过超声波穿孔(或细胞表面的超声诱导瞬时孔)将这些微泡输送到细胞中[24]。 siRNA作为一类非编码RNA分子,通过与特定mRNA序列配对发挥作用,导致mRNA的降解,并增强髓核细胞的生物学活性,促进细胞外基质的合成,是一种潜在的椎间盘退变的治疗方法。Banala等[64]在兔模型中通过单独应用siRNA(Caspase 3和ADAMTS5)和组合siRNA(Caspase 3-ADAMTS5)来检测siRNA在椎间盘退变中的表达效应,结果显示siRNA在下调Caspase 3和ADAMTS5基因表达中的作用是有益的,表明对siRNA的干预能降低椎间盘细胞凋亡的程度,为椎间盘退行性变的再生治疗提供了可能。同时,韩敦富等[65]在研究中表示siRNA可降低大鼠椎间盘细胞对白细胞介素1β预刺激导致的凋亡敏感性。Suzuki等[66]也采用类似的研究,得出了与韩等相一致的结论,并表示RNAi可应用于椎间盘退变和相关疾病的局部治疗。 miRNAs是一种小的非编码RNA分子,内源性表达,可以通过靶向mRNAs进行翻译抑制和分裂来抑制基因表达。椎间盘退变性疾病患者中miRNA表达失调的存在表明miRNA可能在椎间盘退变的发病机制中起重要作用。Zhao等[67]认为椎间盘退变性疾病患者中miRNA表达失调的存在,表明miRNA可能在椎间盘退变的发病机制中起重要作用。Ohrt-Nissen等[68]在14例腰椎间盘退行性变患者的椎间盘中提取RNA,发现有10种miRNA在髓核中表达增高。由此可见,miRNA可作为椎间盘退变的生物标志物。随着研究的深入和新技术的发展,未来的研究可能会确定miRNA在椎间盘退行性疾病发展中的重要性,并实现miRNA在体内的传导。 lncRNA最近已被鉴定为基因表达的重要调节剂,过去5年来,在椎间盘退变中的研究非常活跃,目前,Li等[69]总结了关于失调的lncRNA(例如:RP11-296A18.3,TUG1,HCG18)在椎间盘退变中调节髓核细胞功能中的作用,他们发现,lncRNA或其下游信号通路的特异性调节,可能是开发椎间盘退变新疗法中最有吸引力的方向。 与基因治疗相关的其他疗法:另外再生治疗包括核酸治疗和联合治疗,核酸治疗由于具有在转录或转录后水平抑制基因表达的潜力,而变得日益重要,核酸治疗通过基因改变人类细胞为治疗遗传性疾病提供了极好的机会[22]。因此其可用于治疗各种疾病,其中就包括椎间盘退变相关疾病。联合疗法是使用基于细胞的基因递送进行的替代疗法,其中启动子被遗传结合到干细胞中,以增加软骨形成基因并抑制成骨基因[22]。通过使用联合治疗,可使治疗方法取长补短,减少并发症的发生。 "
| [1]Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP. Matrix Biol. 2014; 40:10-16.[2]Schol J, Sakai D. Cell therapy for intervertebral disc herniation and degenerative disc disease: clinical trials. Int Orthop. 2019;43(3):1011-1025. [3]Liu Y, Yu T, Ma XX, et al. Lentivirus-mediated TGF-beta3, CTGF and TIMP1 gene transduction as a gene therapy for intervertebral disc degeneration in an in vivo rabbit model. Exp Ther Med. 2016;11(4):1399-1404.[4]Navone SE, Marfia G, Giannoni A, et al. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol Histopathol. 2017;32(6):523-542.[5]Anitua E, Padilla S. Biologic therapies to enhance intervertebral disc repair. Regen Med. 2018;13(1):55-72.[6]Lawson LY, Harfe BD. Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip Rev Dev Biol. 2017;6(6):e283-e296.[7]Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448-457.[8]Hemanta D, Jiang XX, Feng ZZ, et al. Etiology for degenerative disc disease. Chin Med Sci J. 2016;31(3): 185-191.[9]Ouyang ZH, Wang WJ, Yan YG, et al. The PI3K/Akt pathway: a critical player in intervertebral disc degeneration. Oncotarget. 2017;8(34):57870-57881.[10]Wang C, Wang W, Yang W, et al. MicroRNAs: a type of novel regulative factor for intervertebral disc degeneration. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016;45(2):170-178.[11]Stergar J, Gradisnik L, Velnar T, et al. Intervertebral disc tissue engineering: a brief review. Bosn J Basic Med Sci. 2019;19(2):130-137.[12]Helm Ii S, Simopoulos TT, Stojanovic M, et al. Effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2017;20(6):447-470.[13]Frost BA, Camarero-Espinosa S, Foster EJ. Materials for the spine: anatomy, problems, and solutions. Materials (Basel). 2019;12(2):253-293.[14]Donnally IC, Varacallo M. Lumbosacral Disc Injuries. StatPearls. Treasure Island (FL). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019.[15]Feng C, Liu H, Yang M, et al. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle. 2016;15(13):1674-1684.[16]Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016; 34(8):1289-1306.[17]Blanquer SB, Grijpma DW, Poot AA. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev. 2015;84:172-187.[18]Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54-67.[19]Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: Roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur Cells Mater. 2015;30:104-117.[20]Vadala G, Russo F, Ambrosio L, et al. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016; 8(5):185-201.[21]Smith LJ, Silverman L, Sakai D, et al. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine, 2018;1(4):e1036-e1049.[22]Sampara P, Banala RR, Vemuri SK, et al. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25(2):67-82.[23]Basso M, Cavagnaro L, Zanirato A, et al. What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskeletal Surg. 2017;101(2):93-104.[24]Dowdell J, Erwin M, Choma T, et al. Intervertebral Disk Degeneration and Repair. Neurosurgery. 2017;80(3S): S46-S54.[25]Sun Y, Leung VY, Cheung KM. Clinical trials of intervertebral disc regeneration: current status and future developments. Int Orthop. 2019;43(4):1003-1010.[26]Kennon J C, Awad ME, Chutkan N, et al. Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomol Concepts. 2018;9(1):43-52.[27]Tan Y, Yao X, Dai Z, et al. Bone morphogenetic protein 2 alleviated intervertebral disc degeneration through mediating the degradation of ECM and apoptosis of nucleus pulposus cells via the PI3K/Akt pathway. Int J Mol Med. 2019;43(1): 583-592.[28]Yang W, Yu XH, Wang C, et al. Interleukin-1beta in intervertebral disk degeneration. Clinica Chim Acta. 2015;450: 262-272.[29]Li P, Zhang R, Gan Y, et al. Effects of osteogenic protein-1 on intervertebral disc regeneration: a systematic review of animal studies. Biomed Pharmacother. 2017;88:260-266.[30]Wang SZ, Chang Q, Lu J, et al. Growth factors and platelet-rich plasma: promising biological strategies for early intervertebral disc degeneration. Int Orthop. 2015;39(5): 927-934.[31]Jia J, Wang SZ, Ma LY, et al. The differential effects of leukocyte-containing and pure platelet-rich plasma on nucleus pulposus-derived mesenchymal stem cells: implications for the clinical treatment of intervertebral disc degeneration. Stem Cells Int. 2018;2018:7162084.[32]Gelalis ID, Christoforou G, Charchanti A, et al. Autologous platelet-rich plasma (PRP) effect on intervertebral disc restoration: an experimental rabbit model. Eur J Orthop Surg Traumatol. 2019;29(3):545-551.[33]Akeda K, Yamada J, Linn ET, et al. Platelet-rich plasma in the management of chronic low back pain: a critical review. J Pain Res. 2019;12:753-767.[34]Feng C, Liu H, Yang Y, et al. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell Physiol Biochem. 2015;35(1):1-16.[35]Tran CM, Shapiro IM, Risbud MV. Molecular regulation of CCN2 in the intervertebral disc: lessons learned from other connective tissues. Matrix Biol. 2013;32(6):298-306.[36]Shen L, Wu Y, Han L, et al. Overexpression of growth and differentiation factor-5 inhibits inflammatory factors released by intervertebral disc cells. Exp Ther Med. 2018;15(4):3603-3608.[37]Zhu J, Xia K, Yu W, et al. Sustained release of GDF5 from a designed coacervate attenuates disc degeneration in a rat model. Acta Biomater. 2019;86:300-311.[38]Sakai D, Schol J. Cell therapy for intervertebral disc repair: clinical perspective. J Orthop Translat. 2017;9:8-18.[39]Vasiliadis ES, Pneumaticos SG, Evangelopoulos DS, et al. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol Med. 2014;20:400-409.[40]Chakravarthy K, Chen Y, He C, et al. Stem cell therapy for chronic pain management: review of uses, advances, and adverse effects. Pain Physician. 2017;20(4):293-305.[41]Nukaga T, Sakai D, Tanaka M, et al. Transplantation of activated nucleus pulposus cells after cryopreservation: efficacy study in a canine disc degeneration model. Eur Cells Mater. 2016;31:95-106.[42]Gantenbein B, Calandriello E, Wuertz-Kozak K, et al. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskelet Disord. 2014;15:422-436.[43]Mochida J, Sakai D, Nakamura Y, et al. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cells Mater. 2015;29:202-212.[44]Xia K, Gong Z, Zhu J, et al. Differentiation of pluripotent stem cells into nucleus pulposus progenitor cells for intervertebral disc regeneration. Curr Stem Cell Res Ther. 2019;14(1): 57-64.[45]Yoshikawa T, Ueda Y, Miyazaki K, et al. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine. 2010;35(11):E475-E480.[46]Kraus P, Lufkin T. Implications for a stem cell regenerative medicine based approach to human intervertebral disk degeneration. Front Cell Dev Biol. 2017;5:17-22.[47]Noriega DC, Ardura F, Hernandez-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945-1951.[48]Fernandez-Moure J, Moore CA, Kim K, et al. Novel therapeutic strategies for degenerative disc disease: Review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018;6:2050312118761674.[49]Mayer JE, Iatridis JC, Chan D, et al. Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013;13(3):299-317.[50]Yue B, Lin Y, Ma X, et al. Effect of Survivin gene therapy via lentivirus vector on the course of intervertebral disc degeneration in an in vivo rabbit model. Mol Med Rep. 2016;14(5):4593-4598.[51]Smith E, Blomberg P. Gene therapy-from idea to reality. Lakartidningen. 2017;114:EWYL.[52]Wehling P, Schulitz KP, Robbins PD, et al. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22(10):1092-1097.[53]Zhang YH, Zhao YL, Li B, et al. Lentivirus is an efficient and stable transduction vector for intervertebral disc cells. World Neurosurg. 2018;111:e348-e354.[54]Lei T, Quan Z, Zhang Y, et al. Transfection of lentivirus-bone morphogenetic protein 2 and lentivirus-inhibitor of differentiation 1 into nucleus pulposus for delaying intervertebral disc degeneration in an in vivo rabbit model. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(1): 73-79.[55]Yue B, Lin Y, Ma X, et al. Survivin-TGFB3-TIMP1 gene therapy via lentivirus vector slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine. 2016; 41(11):926-934.[56]Luo XW, Liu K, Chen Z, et al. Adenovirus-mediated GDF-5 promotes the extracellular matrix expression in degenerative nucleus pulposus cells. J Zhejiang Univ Sci B. 2016;17(1): 30-42.[57]Chu G, Shi C, Wang H, et al. Strategies for annulus fibrosus regeneration: from biological therapies to tissue engineering. Front Bioeng Biotechnol. 2018;6:90-102.[58]Cao D, Quan Z, Jiang D, et al. Effect of adenovirus human bone morphogenetic protein 4 on human degenerative lumbar intervertebral disc cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(12):1442-1447.[59]Ren S, Liu Y, Ma J, et al. Treatment of rabbit intervertebral disc degeneration with co-transfection by adeno-associated virus-mediated SOX9 and osteogenic protein-1 double genes in vivo. Int J Mol Med. 2013;32(5):1063-1068.[60]唐琦,邱立鹏,李东,等.杆状病毒表达载体的应用现状[J].微生物学通报,2018,45(2):442-450.[61]凌同,余黎,白慕群.昆虫杆状病毒表达系统的研究进展与应用[J].微生物学免疫学进展,2014,42(2):70-78.[62]Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541-555.[63]谢丽娜,罗聪,王昌绚,等.可降解的非病毒基因载体的研究进展[J].基因组学与应用生物学,2018,37(6):2668-2671.[64]Banala RR, Vemuri SK, Dar GH, et al. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 2018;19(5):896-904.[65]韩敦富,尹荷珊,王延,等.siRNA可降低IL-1β刺激椎间盘细胞所致凋亡敏感性[J].安徽医科大学学报,2019,54(1):64-68.[66]Suzuki T, Nishida K, Kakutani K, et al. Sustained long-term RNA interference in nucleus pulposus cells in vivo mediated by unmodified small interfering RNA. Eur Spine J. 2009;18(2): 263-270.[67]Zhou X, Chen L, Grad S, et al. The roles and perspectives of microRNAs as biomarkers for intervertebral disc degeneration. J Tissue Eng Regen Med. 2017;11(12):3481-3487.[68]Ohrt-Nissen S, Dossing KB, Rossing M, et al. Characterization of miRNA expression in human degenerative lumbar disks. Connect Tissue Res. 2013;54(3):197-203.[69]Li Z, Li X, Chen C, et al. Long non-coding RNAs in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2018;51(5):e12483-e12490. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Lü Zhen, Bai Jinzhu. A prospective study on the application of staged lumbar motion chain rehabilitation based on McKenzie’s technique after lumbar percutaneous transforaminal endoscopic discectomy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1398-1403. |
| [3] | Gao Yan, Zhao Licong, Zhao Hongzeng, Zhu Yuanyuan, Li Jie, Sang Deen. Alteration of low frequency fluctuation amplitude at brain-resting state in patients with chronic discogenic low back pain [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1160-1165. |
| [4] | Wu Xun, Meng Juanhong, Zhang Jianyun, Wang Liang. Concentrated growth factors in the repair of a full-thickness condylar cartilage defect in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1166-1171. |
| [5] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [6] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [7] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [8] | Luo Xuanxiang, Jing Li, Pan Bin, Feng Hu. Effect of mecobalamine combined with mouse nerve growth factor on nerve function recovery after cervical spondylotic myelopathy surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 719-722. |
| [9] | Nie Huijuan, Huang Zhichun. The role of Hedgehog signaling pathway in transforming growth factor beta1-induced myofibroblast transdifferentiation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 754-760. |
| [10] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [11] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [12] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [13] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [14] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [15] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||