Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (32): 5209-5215.doi: 10.3969/j.issn.2095-4344.1400
Previous Articles Next Articles
Subchondral bone cysts in osteoarthritis: abnormal reconstruction of subchondral bone
Lu Jinwei1, Chen Xi2, Ye Chenyi1, He Rongxin3, Tang Lan1, Yu Yunxian2
-
Online:2019-11-18Published:2019-11-18 -
Contact:Yu Yunxian, MD, Associate professor, Doctoral supervisor, Teaching and Research Section of Epidemiology and Health Statistics, Department of Public Health, School of Medicine of Zhejiang University, Hangzhou 310058, Zhejiang Province, China -
About author:Lu Jinwei, Second Department of Clinical Medicine, School of Medicine of Zhejiang University, Hangzhou 310058, Zhejiang Province, China Chen Xi, Master, Teaching and Research Section of Epidemiology and Health Statistics, Department of Public Health, School of Medicine of Zhejiang University, Hangzhou 310058, Zhejiang Province, China Lu Jinwei and Chen Xi contributed equally to this study.
CLC Number:
Cite this article
Lu Jinwei, Chen Xi, Ye Chenyi, He Rongxin, Tang Lan, Yu Yunxian. Subchondral bone cysts in osteoarthritis: abnormal reconstruction of subchondral bone[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(32): 5209-5215.
share this article
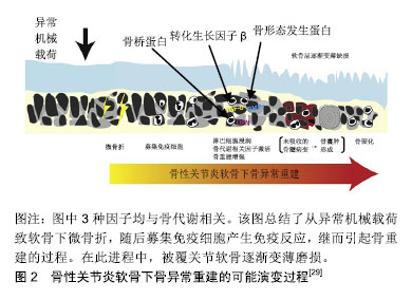
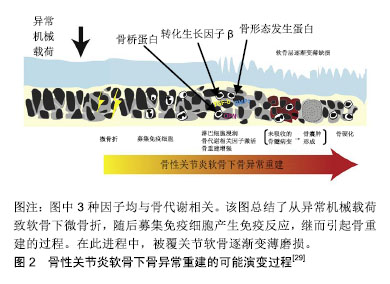
2.1 软骨下骨囊肿的流行病学 软骨下骨囊肿在骨性关节炎中常见,大约50%膝骨性关节炎患者有软骨下骨囊肿,在健康人中亦有13.7%出现该病变[11],伴随骨性关节炎进展,软骨下骨囊肿可增大、新发[12]。类风湿关节炎、强直性脊柱炎、股骨头坏死亦可见软骨下骨囊肿[13-15]。 2.2 软骨下骨囊肿的临床意义 在疼痛症状方面,Takegami等[16]发现髋关节软骨下骨囊肿与目测类比疼痛评分及生活质量评分显著相关,股骨头及髋臼同时出现软骨下骨囊肿有着最高的目测类比疼痛评分,股骨头及髋臼软骨下骨囊肿的最大直径与目测类比疼痛评分独立相关。Burnett等[17]研究发现,伴有重度夜间痛的膝关节骨性关节炎患者,其软骨下骨囊肿的程度与无夜间痛的患者相比更严重。 在关节结构进展方面,Raynauld等[18]纳入107例症状性膝关节骨性关节炎患者,随访2年的回顾性分析表明,股骨内侧髁的软骨下骨囊肿的直径与软骨量呈显著负相关。Tanamas等[11]纳入132例症状性膝关节骨性关节炎患者的前瞻性随访研究发现,与基线仅存在软骨下骨髓病变和无病变者相比,基线软骨下骨髓病变+软骨下骨囊肿的患者的胫骨内侧平台软骨丢失明显增多、胫骨内外侧平台软骨量明显降低。McErlain等[19]通过对早期膝关节骨性关节炎患者进行有限元分析,表明软骨下骨囊肿使毗邻的软骨下骨平均应力增加约2倍,软骨下骨囊肿直径与该平均应力呈正相关。作者认为由软骨下骨囊肿所致异常的应力分布可加速骨性关节炎进程,加剧疼痛和功能障碍。Kastelein等[20]通过6年的队列研究发现,膝关节内侧间室软骨下骨囊肿是更高Kellgren-Lawrence分级的危险因素,OR=11.7。 之于手术,Tanner等[21]通过对75例肩关节骨性关节炎行全肩关节置换患者5-8年的随访研究发现,术前存在中度-重度软骨下骨囊肿者与无-轻度软骨下骨囊肿者相比有着更高的手术翻修率(12.5% vs. 0),翻修原因均为关节盂假体松动,作者认为术前通过CT评估软骨下骨囊肿的程度可帮助评估手术失败的风险。 2.3 软骨下骨囊肿的成因假说 软骨下骨囊肿的成因尚无定论,目前主要分为“滑液入侵假说”和“创伤性骨坏死假说”[22-24]。 1953年Landells[25]发现软骨下骨囊肿通过裂隙与关节腔相通,因此认为其并不是原发于软骨下骨病变,而是由于关节滑液等通过裂隙进入软骨下骨,造成软骨下骨小梁的不全骨折及移位形成囊肿的壁,骨小梁的推移引起了局部骨硬化的表现,同时由于空腔的形成使得应力改变,促进囊肿壁的新骨形成,且最终由于纤维组织增生、新骨形成等原因可自发封闭连通关节腔的通道,即“滑液入侵假说”。 1955年Rhaney等[26]认为软骨下骨囊肿的形成并不是“滑液入侵假说”,而是由于没有正常的关节软骨的保护,强大外力传递至软骨下骨,通过血管损伤、缺血等环节引起骨坏死,进而在骨吸收的过程中形成含有液体的腔隙,即“创伤性骨坏死假说” 。 2006年一项纳入32例骨性关节炎患者平均随访17.52个月的回顾性临床研究表明,基于MRI检查,92% 软骨下骨囊肿起源于软骨下骨髓病变的信号区域,且软骨下骨囊肿的发展通常伴随着软骨下骨髓病变大小的改变,该研究提示软骨下骨髓病变和软骨下骨囊肿可能存在密切联系[27]。2010年一项纳入1 283例膝关节骨性关节炎高危患者(超重、肥胖、膝痛、晨僵、膝关节外伤史、膝关节手术史)的随访30个月的大规模前瞻性临床研究表明,在校正了其他混杂因素后,软骨下骨髓病变与软骨下骨囊肿显著相关,二者在时间上有先后关系,在空间上有一致性,前者是后者的预测因素,表明二者可能是同一病理过程的不同阶段,随后有学者称软骨下骨髓病变为“软骨下骨囊肿前病变”[28-29]。另一方面,由于此时毗邻的关节软骨损伤并未累及全层,即关节滑液尚无法进入软骨下骨中,因而不支持“滑液入侵假说”,加之已有观点认为软骨下骨髓病变是由外力引起的软骨下骨不全骨折或微骨折所致,并可进展为骨性关节炎,所以上述研究更倾向于“创伤性骨坏死”假说[28,30]。 然而Chan等[31]认为,尽管以上2种假说能部分解释负重区域软骨下骨囊肿的成因,却并不能解释分布于非负重区域的软骨下骨囊肿,缘其研究结果:在非承重部位如胫骨髁间棘或外侧间室亦可出现软骨下骨囊肿,此时其被覆的软骨并无肉眼可见的损伤。由此产生第3种假说:非负重区域软骨下骨囊肿可能与血管老化和内皮细胞功能障碍导致缺血性事件进而继发骨量减少有关(即“缺血性骨量减少假说”)。然而这一假说较少见,尚无临床研究支持或反对。 2017年Weber等[29]总结既往的研究,认为软骨下骨囊肿在骨性关节炎中可能的演变机制为:异常的机械载荷作用于软骨并传导至软骨下骨引起软骨下骨微骨折,随后募集免疫细胞(单核、多核细胞),激活骨生长因子,启动软骨下骨异常重建,该期间形成软骨下骨髓病变,其中未吸收者形成软骨下骨囊肿,至终末期软骨下骨硬化。整个进程中,关节软骨损伤逐渐加重,见图2。"
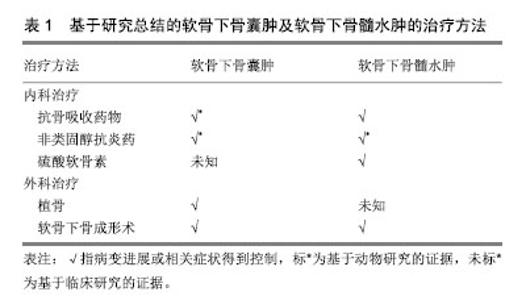
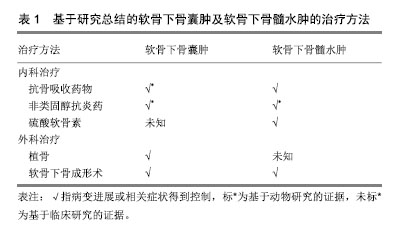
2.4 软骨下骨囊肿病理表现 肉眼观为灰色凝胶状区域,通常多发,大小从1.0-2.5 cm不等,超过1 cm的囊肿只在股骨头可见,体积分数与年龄和性别无明显关系,通常为圆形、梨形、卵形、不规则形,可单房或多房。髋关节骨性关节炎患者的软骨下骨囊肿发生的部位通常位于髋臼或股骨头,通过斜而曲折的通道与关节腔相通,有些较大的囊肿可以延伸至股骨颈,关节面的碎骨片可经该通道进入囊肿[25]。髋臼处软骨下骨囊肿总是发生于髂前下棘以及髋臼软骨和髋臼唇的交界处(即承重最大的部位)。囊肿可以长时间保持稳定或者短时间内急速增大,患者可以无症状或表现出经典的骨性关节炎症状。当囊肿壁硬化和周围硬化骨发生骨折或囊肿腔塌陷时,往往会加剧骨性关节炎的症状。囊肿内包含寡细胞的纤维组织并在囊肿周围形成硬化骨,内含物在血液供应消失后发生黏液变性,形成影像学上透亮的区域[32]。 软骨下骨囊肿的区域不仅可见活化的破骨细胞,亦可见活化的成骨细胞[33]。软骨下骨囊肿的空洞形成、扩大与破骨细胞造成的骨吸收相关,亦有文献将其称为软骨下骨吸收陷窝[34-36]。Sabokbar等[37]对大鼠骨肉瘤细胞(UMR106)和软骨下骨囊肿来源的巨噬细胞的联合培养14 d后,发现其表达抗酒石酸酸性磷酸酶、玻连蛋白受体,并形成许多骨吸收腔隙,表明巨噬细胞分化成破骨细胞。该结果提示巨噬细胞向破骨细胞的分化参与了骨性关节炎患者软骨下骨囊肿的增大。 动物实验表明,在软骨下骨囊肿中,炎症因子前列腺素E2明显升高[38],而已有研究证明高水平前列腺素E2与骨吸收相关[39],因此可能前列腺素E2通过促进骨吸收参与软骨下骨囊肿的进程。 综上所述,软骨下骨囊肿的病理生理机制可能是包括炎症因子在内的各种因素引起的骨吸收及继发的反应性成骨[40-41],该病理过程可能较骨赘及软骨下骨髓病变更为典型地反映骨性关节炎软骨下骨的异常重建。 2.5 软骨下骨囊肿的影像学评估 常规X射线片对发现软骨下骨囊肿的价值有限,Audrey等[42]纳入806例保守治疗无效的症状性膝关节骨性关节炎患者,回顾性分析发现,相比骨性关节炎的其他影像学征象(关节腔狭窄99.5%,骨赘形成98.1%,软骨下骨硬化接近90%),软骨下骨囊肿的X射线表现只见于30.6%的患者,作者认为软骨下骨囊肿不是骨性关节炎的主要影像学特征。但其他研究表明,使用负重45°屈膝后前位膝关节摄片比普通负重前后位膝关节摄片诊断膝关节骨性关节炎更准确[43];而此方位摄片+负重前后位膝关节摄片比单用负重前后位膝关节摄片明显提高膝骨性关节炎的软骨下骨囊肿的检出率[44]。 CT和MRI对发现软骨下骨囊肿更有帮助。现在已有研究报道在骨性关节炎早期(Kellgren-Lawrence分级≤2)的患者中,通过CT可发现软骨下骨囊肿[19]。而站立位负重CT比双足外旋10°站立固定20°-30°屈曲后前位膝关节X射线片发现软骨下骨囊肿更优,灵敏度100% vs. 10%[45]。Javaid等[46]开展的研究纳入158例X射线片无骨性关节炎征象的骨性关节炎高危患者,MRI示27%的患者在基线即存在软骨下骨囊肿。Guermazi等[47]开展的骨性关节炎研究纳入X射线片上Kellgren-Lawrence分级0级的710例患者,MRI示25%的患者存在软骨下骨囊肿。但CT和MRI的价格均比X射线片昂贵,且摄片时间更长,或许是目前临床仍主要以X射线片为骨性关节炎诊断及分级标准的原因[48]。 基于Chen等[12]研究证实,软骨下骨囊肿区域骨矿物密度显著低于无软骨下骨囊肿组。Burnett等[49]曾认为经定量CT或双能X射线骨矿物密度检测软骨下松质骨低骨矿物密度可能提示软骨下骨囊肿的存在,但其在2019年的定量计算机断层扫描研究中得出了截然相反的结果:软骨下骨囊肿的大小和数量与软骨下骨矿物密度正相关[50]。因而以骨矿物密度评估软骨下骨囊肿的方法仍有待探索。 2.6 软骨下骨囊肿的治疗 软骨下骨囊肿的治疗包括软骨下骨囊肿的治疗和软骨下骨囊肿前病变(即软骨下骨髓病变)的治疗。 2.6.1 软骨下骨囊肿的治疗 软骨下骨囊肿的治疗指征尚无定论,Lee等[51]认为出现进展的、影响关节面结构的软骨下骨囊肿需要治疗,治疗早期的伴有大的、多发软骨下骨囊肿的骨性关节炎可延缓疾病进展 。 动物研究表明,降钙素可显著减少软骨下骨囊肿的数量/体积,作者归因于其抑制软骨下骨异常重建从而改善了软骨下骨小梁微结构[52]。Pelletier等[53]以前交叉韧带切除建立犬膝关节骨性关节炎模型,并予以非类固醇抗炎药治疗8周,结果表明,对照组(无治疗)骨性关节炎犬的软骨下骨空腔增多,而实验组骨性关节炎犬的软骨下骨形态类似于健康犬。而2018年基于雌性骨性关节炎大鼠的动物研究表明,使用环氧化酶2抑制剂缓释剂型单次关节腔内注射后16周,软骨下骨囊肿显著改善,但与健康组相比差异仍有显著性意义,作者认为该疗效的机制是环氧化酶2抑制剂抑制“炎症相关通路(如核因子κB)所致的破骨细胞活化及继发的骨吸收”[54]。 Lee等[51]在2015年对2例伴软骨下骨囊肿的早期髋关节骨性关节炎患者行同种异体骨植入术,术后植骨长入良好,关节功能恢复良好。而Ohishi等[55]在2016年对于晚期右膝骨性关节炎伴巨大软骨下骨囊肿的患者行限制性假体全膝关节置换+软骨下骨囊肿打压植骨则同样取得了积极效果。 Jacobs[56]早在1963年便提出位于股骨的软骨下骨囊肿可以用远端截骨术以重组关节表面和重新分散过高的力学载荷,以达到减轻疼痛,减少囊肿的大小与数量和改善关节功能的作用。但Mechlenburg等[57]对26例髋关节发育不良继发骨性关节炎患者行髋关节周围截骨术后的10年随访中,发现软骨下骨囊肿的平均直径无显著变化,造成试验结果阴性的可能原因是样本量过小,失访率较高。 Iijima等[58]对12周龄大鼠以内侧半月板脱稳定术建立膝骨性关节炎模型,后进行共4周,5次/周,30 min/次,12 m/min的慢走干预,经显微CT证实,尽管未达到显著性差异(P=0.06),慢走已表现出抑制软骨下骨囊肿进展的趋势,提示术后慢走可能对软骨下骨囊肿产生积极效果。 Bertrand[59]对13例症状性软骨下骨囊肿患者行CT和透视引导下的磷酸钙骨水泥填充术(即软骨下骨成形术),平均随访22个月后,12例患者疼痛显著改善,所有患者无早期与晚期并发症。与传统的植骨术式相比,骨水泥填充术效果显著,侵入性小,或许可以作为有症状软骨下骨囊肿患者的手术方式首选。 2.6.2 软骨下骨髓病变的治疗 曾有观点认为大多数的软骨下骨髓病变会自行吸收,呈一过性,因此可不干预,但Carrino等[27]的研究发现未经治疗的软骨下骨髓病变(有/无症状)患者,其中57%会发生进展,但究竟何种软骨下骨髓病变患者需要干预,作者并未阐明。近年的研究表明,发生在膝关节的伴有疼痛症状的软骨下骨髓病变(持续30 d-1年)难以自行吸收,因而可能需要干预[60]。 2004年一项纳入818例绝经后症状性膝骨性关节炎妇女的临床研究表明,在使用双膦酸盐平均1.8(<1-4)年后,膝关节软骨下骨髓病变程度经MRI检测好转(已校正混杂因素)[61]。2010年Jones等[62]通过切除大鼠膝关节内侧副韧带、前交叉韧带、内侧半月板建立创伤性骨性关节炎模型,术后使用非类固醇抗炎药+双膦酸盐8周比未治疗组的软骨下骨髓病变区域的T2信号降低,且与假手术组差异无显著性意义,表明其具有改善软骨下骨髓病变的效果。2011年一项多中心、随机双盲对照临床试验纳入69例骨性关节炎患者,分别予以硫酸软骨素800 mg 1次/d(35例)和安慰剂(34例),随访12个月时,胫骨外侧平台和股骨外侧髁的软骨下骨髓病变评分显著降低[63]。 2016年Cohen等[64]通过对66例有软骨下骨髓病变的骨性关节炎患者实行软骨下骨成形术后进行回顾性研究发现,87.8%的患者目测类比疼痛评分降低。术后随访2年患者的目测类比疼痛评分平均降低了4.5分(以2分为最小有意义变化值)。同时有60例患者接受了至少2年随访,其中42例(70%)患者未进一步行关节置换术,余30%患者延长关节置换时间平均3.1年。综上所述,软骨下骨成形术对于骨性关节炎的软骨下骨髓病变的短中期疗效确切。软骨下骨成形术在保留关节的前提下减轻了疼痛,改善了关节功能,适合年纪较轻,运动需求较高且不希望接受关节置换术的患者。 2018年Brimmo等[65]学者通过犬骨性关节炎模型证实,软骨下骨成形术后一两年关节疼痛、肿胀以及关节功能改善均优于对照组,且安全性良好。但无论是人体试验抑或动物实验,在软骨下骨成形术术后早期阶段,仍可出现轻到中度的疼痛及较对照组功能障碍加重的现象,Brimmo等[65-66]猜测这可能与填充物过度注入及填充物渗出有关。 尽管如此,目前软骨下骨成形术仍是一种新兴的治疗骨性关节炎软骨下骨髓病变的富有前景的手术疗法,其对于软骨下骨髓病变的结构性进展及预防软骨下骨囊肿的疗效仍需要后续试验证明。 总结软骨下骨囊肿及软骨下骨髓病变的治疗见表1。"
| [1]Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.[2]Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134-138.[3]Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.[4]Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8(6):225-235.[5]Everhart JS, Abouljoud MM, Kirven JC, et al. Full-thickness cartilage defects are important independent predictive factors for progression to total knee arthroplasty in older adults with minimal to moderate osteoarthritis: data from the osteoarthritis initiative. J Bone Joint Surg Am. 2019;101(1):56-63.[6]Chen Y, Hu Y, Yu YE, et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J Bone Miner Res. 2018;33(2):316-327.[7]Okada K, Yamaguchi S, Sato Y, et al. Comparison of meniscal extrusion and osteophyte formation at the intercondylar notch as a predictive biomarker for incidence of knee osteoarthritis-Data from the Osteoarthritis Initiative. J Orthop Sci. 2019;24(1):121-127.[8]Xu ZW, Chen TM, Luo J, et al. Cartilaginous Metabolomic Study Reveals Potential Mechanisms of Osteophyte Formation in Osteoarthritis. J Proteome Res. 2017;16(4):1425-1435.[9]Zhu QC, Xu JH, Wang K, et al. Associations between systemic bone mineral density, knee cartilage defects and bone marrow lesions in patients with knee osteoarthritis. International J Rheum Dis. 2018; 21(6):1202-1210.[10]Alliston T, Hernandez CJ, Findlay DM, et al. Bone marrow lesions in osteoarthritis: What lies beneath. J Orthop Res. 2018;36(7):1818-1825.[11]Tanamas SK, Wluka A, Pelletier JP, et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis Res Ther. 2010;12(2):R58.[12]Chen Y, Wang T, Guan M, et al. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr Cartilage. 2015;23(12):2174-2183.[13]Ostrowska M, Maslinski W, Prochorec-Sobieszek M, et al. Cartilage and bone damage in rheumatoid arthritis. Reumatologia. 2018;56(2): 111-120.[14]Wick MC, Weiss RJ, Jaschke W, et al. Erosions are the most relevant magnetic resonance imaging features in quantification of sacroiliac joints in ankylosing spondylitis. J Rheumatol. 2010;37(3):622-627.[15]Lakhotia D, Swaminathan S, Shon WY, et al. Healing process of osteonecrotic lesions of the femoral head following transtrochanteric rotational osteotomy: a computed tomography-based study. Clin Orthop Surg. 2017;9(1):29-36.[16]Takegami Y, Seki T, Higuchi Y, et al. Independent association of joint space narrowing, cyst formation and health-related quality of life of patients with hip osteoarthritis in Japan. J Orthop Sci. 2017;22(6): 1096-1101.[17]Burnett WD, Kontulainen SA, McLennan CE, et al. Knee osteoarthritis patients with severe nocturnal pain have altered proximal tibial subchondral bone mineral density. Osteoarthr Cartilage. 2015;23(9): 1483-1490.[18]Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis. 2008; 67(5):683-688.[19]McErlain DD, Milner JS, Ivanov TG, et al. Subchondral cysts create increased intra-osseous stress in early knee OA: A finite element analysis using simulated lesions. Bone. 2011;48(3):639-646.[20]Kastelein M, Luijsterburg PA, Koster IM, et al. Knee osteoarthritis in traumatic knee symptoms in general practice: 6-year cohort study. BMJ Open Sport Exerc Med. 2016;2(1): e000153.[21]Tanner G, Simon P, Sellers T, et al. Total shoulder arthroplasty with minimum 5-year follow-up: does the presence of subchondral cysts in the glenoid increase risk of failure? J Shoulder Elbow Surg. 2018;27(5): 794-800.[22]Casey G. Arthritis: joints inflamed. Nurs N Z. 2015;21(5):20-24.[23]Maruotti N, Corrado A, Cantatore FP. Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol. 2017;232(11):2957-2963.[24]Jones G. What's new in osteoarthritis pathogenesis? Intern Med J. 2016;46(2):229-236.[25]Landells JW. The bone cysts of osteoarthritis. J Bone Joint Surg Br. 1953;35-B(4):643-649.[26]Rhaney K, Lamb DW. The cysts of osteoarthritis of the hip - a radiological and pathological study. J Bone Joint Surg. 1955;37(4): 663-675.[27]Carrino JA, Blum J, Parellada JA, et al. MRI of bone marrow edema- like signal in the pathogenesis of subchondral cysts. Osteoarthr Cartilage. 2006;14(10):1081-1085.[28]Crema MD, Roemer FW, Zhu YY, et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: detection with mr imaging-the most study. Radiology. 2010;256(3):855-862.[29]Weber A, Chan PMB, Wen C. Do immune cells lead the way in subchondral bone disturbance in osteoarthritis? Prog Biophys Mol Biol. 2017. pii: S0079-6107(17)30181-5.[30]Bonadio MB, Ormond AG, Helito CP, et al. Bone Marrow Lesion: Image, Clinical Presentation, and Treatment. Magnetic Resonance Insights. 2017;10:1-6.[31]Chan PMB, Wen CY, Yang WC, et al. Is subchondral bone cyst formation in non-load-bearing region of osteoarthritic knee a vascular problem? Med Hypotheses. 2017;109:80-83.[32]Stark DD, Genant HK, Spring DB. Primary cystic arthrosis of the hip. Skeletal Radiol. 1984;11(2):124-127.[33]Chiba K, Nango N, Kubota S, et al. Relationship between microstructure and degree of mineralization in subchondral bone of osteoarthritis: a synchrotron radiation microCT study. J Bone Miner Res. 2012;27(7):1511-1517.[34]Li B, Marshall D, Roe M, et al. The electron microscope appearance of the subchondral bone plate in the human femoral head in osteoarthritis and osteoporosis. J Anat. 1999;195(Pt 1):101-110.[35]Botter SM, van Osch GJ, Waarsing JH, et al. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthritis Cartilage. 2008;16(4):506-514.[36]McErlain DD, Ulici V, Darling M, et al. An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis. Arthritis Res Ther. 2012;14(1):R26.[37]Sabokbar A, Crawford R, Murray DW, et al. Macrophage-osteoclast differentiation and bone resorption in osteoarthrotic subchondral acetabular cysts. Acta Orthop Scand. 2000;71(3):255-261.[38]Rechenberg B, Guenther H, McIlwraith CW, et al. Fibrous tissue of subchondral cystic lesions in horses produce local mediators and neutral metalloproteinases and cause bone resorption in vitro. Vet Surg. 2000;29(5):420-429.[39]Pelletier JP, Lajeunesse D, Reboul P, et al. Diacerein reduces the excess synthesis of bone remodeling factors by human osteoblast cells from osteoarthritic subchondral bone. J Rheumatol. 2001; 28(4):814-824.[40]Chiba K, Burghardt AJ, Osaki M, et al. Three-dimensional analysis of subchondral cysts in hip osteoarthritis: an ex vivo HR-pQCT study. Bone. 2014;(66):140-145.[41]Li G, Ma Y, Cheng TS, et al. Identical subchondral bone microarchitecture pattern with increased bone resorption in rheumatoid arthritis as compared to osteoarthritis. Osteoarthritis Cartilage. 2014; 22(12):2083-2092.[42]Audrey HX, Abd Razak HR, Andrew TH. The truth behind subchondral cysts in osteoarthritis of the knee. Open Orthop J. 2014;8:7-10.[43]Rosenberg TD, Paulos LE, Parker RD, et al. The forty-five-degree posteroanterior flexion weight-bearing radiograph of the knee. J Bone Joint Surg Am. 1988;70(10):1479-1483.[44]Babatunde OM, Danoff JR, Patrick DA, et al. The combination of the tunnel view and weight-bearing anteroposterior radiographs improves the detection of knee arthritis. Arthritis. 2016;2016:9786924.[45]Segal NA, Nevitt MC, Lynch JA, et al. Diagnostic performance of 3D standing CT imaging for detection of knee osteoarthritis features. Phys Sportsmed. 2015;43(3):213-220.[46]Javaid MK, Lynch JA, Tolstykh I, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthritis Cartilage. 2010;18(3):323-328.[47]Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345.[48]Guermazi A, Hunter DJ, Roemer FW. Plain radiography and magnetic resonance imaging diagnostics in osteoarthritis: validated staging and scoring. J Bone Joint Surg Am. 2009;91 Suppl 1:54-62.[49]Burnett WD, Kontulainen SA, McLennan CE, et al. Response to Letter to the Editor: 'Is subchondral bone mineral density associated with nocturnal pain in knee osteoarthritis patients? Osteoarthritis Cartilage. 2015;23(12):2299-2301.[50]Burnett WD, Kontulainen SA, McLennan CE, et al. Knee osteoarthritis patients with more subchondral cysts have altered tibial subchondral bone mineral density. BMC Musculoskelet Disord. 2019;20(1):14. [51]Lee GS, Hwang DS, Kang C, et al. Arthroscopic treatment of subchondral bony cyst in early osteoarthritis of the hip joint using allogeneic bone graft: a report of two cases. Hip Pelvis. 2015;27(2):110-114.[52]Papaioannou NA, Triantafillopoulos IK, Khaldi L, et al. Effect of calcitonin in early and late stages of experimentally induced osteoarthritis. A histomorphometric study. Osteoarthritis Cartilage. 2007;15(4):386-395.[53]Pelletier JP, Lajeunesse D, Jovanovic DV, et al. Carprofen simultaneously reduces progression of morphological changes in cartilage and subchondral bone in experimental dog osteoarthritis. J Rheumatol. 2000;27(12):2893-2902.[54]Tellegen AR, Rudnik-Jansen I, Pouran B, et al. Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv. 2018;25(1): 1438-1447.[55]Ohishi T, Takahashi M, Suzuki D, et al. Giant intraosseous cyst in an osteoarthritic knee. Orthopedics. 2016;39(6):e1193-e1196.[56]Jacobs P. Primary cystic arthrosis of the hip. Br J Radiol. 1963;(36): 129-134.[57]Mechlenburg I, Nyengaard JR, Gelineck J, et al. Cartilage Thickness and Cyst Volume Are Unchanged 10 Years After Periacetabular Osteotomy in Patients Without Hip Symptoms. Clin Orthop Relat Res. 2015;473(8):2644-2649.[58]Iijima H, Aoyama T, Ito A, et al. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthritis Cartilage. 2015;23(9):1563-1574.[59]Bertrand AS. Percutaneous injection of bone cement (cementoplasty) for the treatment of symptomatic subchondral cysts. J Cell Sci Ther. 2014.[60]Collins JA, Beutel BG, Strauss E, et al. Bone Marrow Edema: Chronic Bone Marrow Lesions of the Knee and the Association with Osteoarthritis. Bull Hosp Jt Dis. 2016;74(1):24-36.[61]Laslett LL, Dore DA, Quinn SJ, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis. 2012;71(8):1322-1328.[62]Jones MD, Tran CW, Li GA, et al. In vivo microfocal computed tomography and micro-magnetic resonance imaging evaluation of antiresorptive and antiinflammatory drugs as preventive treatments of osteoarthritis in the rat. Arthritis Rheum. 2010;62(9):2726-2735.[63]Wildi LM, Raynauld JP, Martel-Pelletier J, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011;70(6):982-989.[64]Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(7):555-563.[65]Brimmo OA, Bozynski CC, Cook CR, et al. Subchondroplasty for the treatment of post-traumatic bone marrow lesions of the medial femoral condyle in a pre-clinical canine model. J Orthop Res. 2018;36(10): 2709-2717.[66]Chatterjee D, McGee A, Strauss E, et al. Subchondral calcium phosphate is ineffective for bone marrow edema lesions in adults with advanced osteoarthritis. Clin Orthop Relat Res. 2015;473(7):2334-2342.[67]中华医学会骨科学分会关节外科学组. 骨关节炎诊疗指南(2018年版) [J]. 中华骨科杂志, 2018, 38(12): 705-715.[68]Gao L, Goebel LKH, Orth P, et al. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018;11(6). pii: dmm034280. [69]Sanchez M, Delgado D, Sanchez P, et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: a pilot study. Biomed Res Int. 2016;2016:4868613. |
| [1] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [2] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [3] | Peng Zhihao, Feng Zongquan, Zou Yonggen, Niu Guoqing, Wu Feng. Relationship of lower limb force line and the progression of lateral compartment arthritis after unicompartmental knee arthroplasty with mobile bearing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1368-1374. |
| [4] | Yuan Jiawei, Zhang Haitao, Jie Ke, Cao Houran, Zeng Yirong. Underlying targets and mechanism of Taohong Siwu Decoction in prosthetic joint infection on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1428-1433. |
| [5] | Liu Xiangxiang, Huang Yunmei, Chen Wenlie, Lin Ruhui, Lu Xiaodong, Li Zuanfang, Xu Yaye, Huang Meiya, Li Xihai. Ultrastructural changes of the white zone cells of the meniscus in a rat model of early osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1237-1242. |
| [6] | Liu Xin, Yan Feihua, Hong Kunhao. Delaying cartilage degeneration by regulating the expression of aquaporins in rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 668-673. |
| [7] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [8] | Cao Xuhan, Bai Zixing, Sun Chengyi, Yang Yanjun, Sun Weidong. Mechanism of “Ruxiang-Moyao” herbal pair in the treatment of knee osteoarthritis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 746-753. |
| [9] | Li Yonghua, Feng Qiang, Tan Renting, Huang Shifu, Qiu Jinlong, Yin Heng. Molecular mechanism of Eucommia ulmoides active ingredients treating synovitis of knee osteoarthritis: an analysis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 765-771. |
| [10] | Song Shan, Hu Fangyuan, Qiao Jun, Wang Jia, Zhang Shengxiao, Li Xiaofeng. An insight into biomarkers of osteoarthritis synovium based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 785-790. |
| [11] | Deng Zhenhan, Huang Yong, Xiao Lulu, Chen Yulin, Zhu Weimin, Lu Wei, Wang Daping. Role and application of bone morphogenetic proteins in articular cartilage regeneration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 798-806. |
| [12] | Liao Qing, Li Baojian, Li Yang, Xu Taixiang, Zeng Jing, Zhang Zhenzhen, Liu Gang. Low-intensity pulsed ultrasound mediates bone marrow mesenchymal stem cells to promote osteoarthritis cartilage repair [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4950-4955. |
| [13] | Cui Xiaoyan. Treatment of knee osteoarthritis with human umbilical cord-derived mesenchymal stem cells combined with sodium hyaluronate injection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4956-4963. |
| [14] | Ling Huajun, Wang Qiyou, Lin Weiwen, Luo Penggang. Bone marrow mesenchymal stem cell derived exosomes delay the occurrence and development of osteoarthritis through cartilage protection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4964-4969. |
| [15] | Zheng Li, Li Dadi, Hu Weifan, Tang Jinlong, Zhao Fengchao. Risk assessment of contralateral knee arthroplasty after unilateral total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 374-379. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||