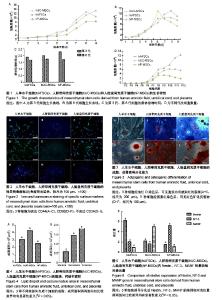
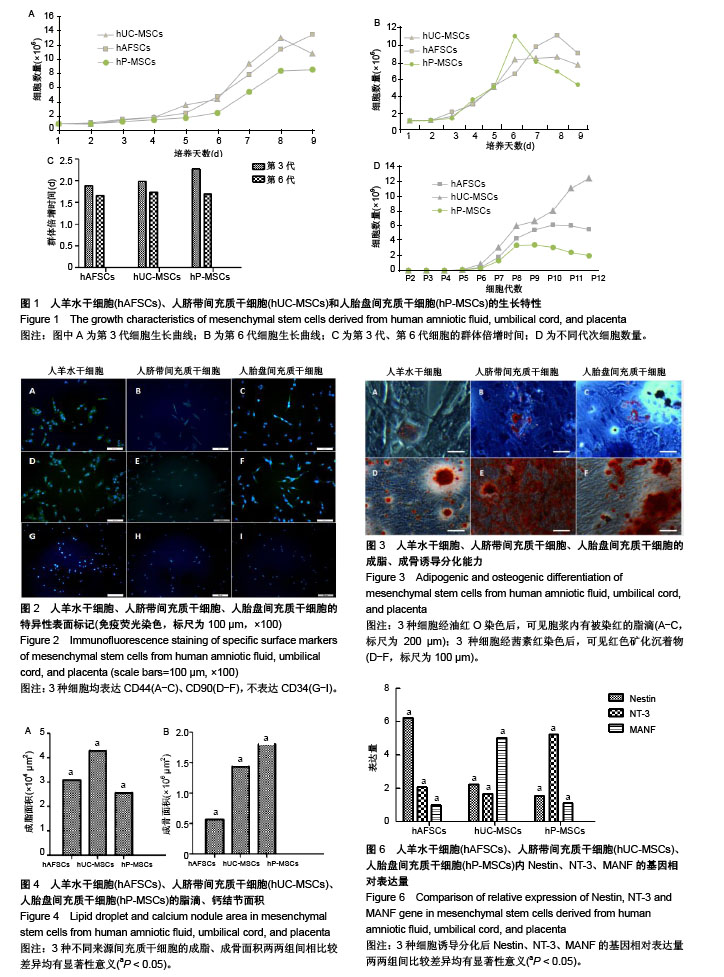
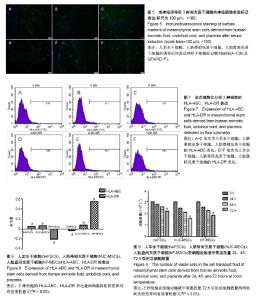
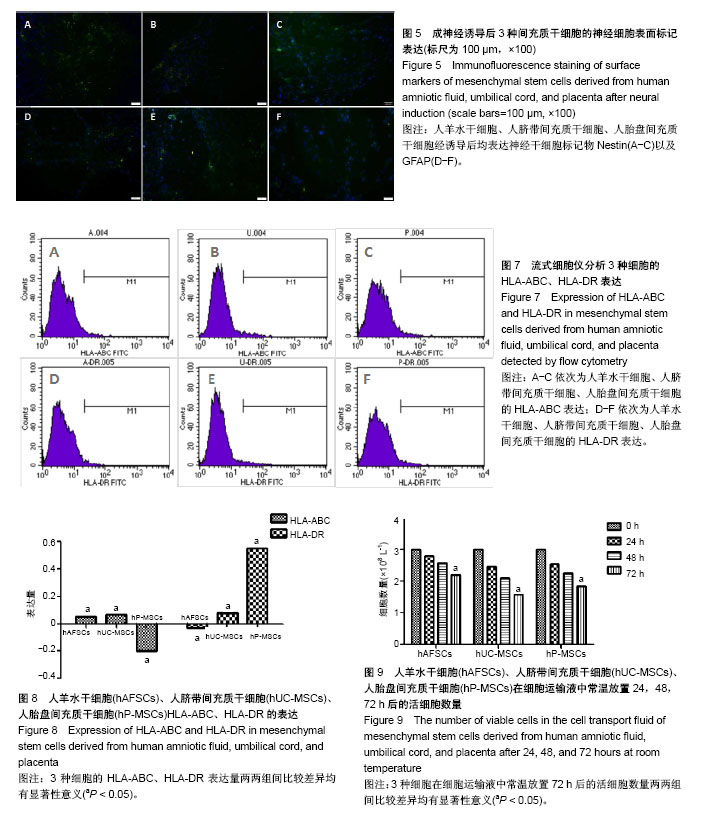
| [1] Macrin D, Joseph JP, Pillai AA, et al. Eminent Sources of Adult Mesenchymal Stem Cells and Their Therapeutic Imminence.Stem Cell Rev. 2017;13(6):741-756.[2] Gholizadeh-Ghaleh Aziz S, Pashaei-Asl F, Fardyazar Z, et al. Isolation, Characterization, Cryopreservation of Human Amniotic Stem Cells and Differentiation to Osteogenic and Adipogenic Cells.PLoS One. 2016;11(7):e0158281.[3] Bonaventura G, Chamayou S, Liprino A, et al. Different Tissue-Derived Stem Cells: A Comparison of Neural Differentiation Capability.PLoS One. 2015;10(10):e0140790.[4] Wang LT, Ting CH, Yen ML, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials.J Biomed Sci. 2016;23(1):76.[5] Collins E, Gu F, Qi M, et al. Differential efficacy of human mesenchymal stem cells based on source of origin.J Immunol. 2014;193(9):4381-4390.[6] Li X, Bai J, Ji X, et al. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34(3):695-704.[7] Janowski M, Lyczek A, Engels C, et al. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation.J Cereb Blood Flow Metab. 2013; 33(6):921-927.[8] Araújo AB, Salton GD, Furlan JM, et al. Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord.Cytotherapy. 2017;19(5):577-585.[9] Rui K, Lin X, Tian J, et al. Ecto-mesenchymal stem cells: a new player for immune regulation and cell therapy.Cell MolImmunol. 2018;15(1):82-84.[10] Barry F, Murphy M.Mesenchymal stem cells in joint disease and repair.Nat Rev Rheumatol. 2013;9(10):584-594.[11] Mareschi K, Castiglia S, Sanavio F, et al. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. ExpHematol. 2016;44(2):138-150.[12] Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, et al. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects.Cryobiology. 2015;71(2):181-197.[13] Danišovi? ?, Bohá? M, Zamborský R, et al. Comparative analysis of mesenchymal stromal cells from different tissue sources in respect to articular cartilage tissue engineering.Gen PhysiolBiophys. 2016;35(2):207-214.[14] Martinelli D, Pereira RC, Mogni M, et al. A humanized system to expand in vitro amniotic fluid-derived stem cells intended for clinical application.Cytotherapy. 2016;18(3):438-451.[15] Pu L, Meng M, Wu J, et al. Compared to the amniotic membrane, Wharton's jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration.Stem Cell Res Ther. 2017;8(1):72.[16] Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC.Cell Commun Signal. 2011;9:12.[17] von Bahr L, Sundberg B, Lönnies L, et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy.Biol Blood Marrow Transplant. 2012;18(4):557-564.[18] Xiao B, Rao F, Guo ZY,et al.Extracellular matrix from human umbilical cord-derived mesenchymal stem cells as a scaffold for peripheral nerve regeneration.Neural Regen Res. 2016; 11(7):1172-1179.[19] Mareschi K, Ferrero I, Rustichelli D, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow.J Cell Biochem. 2006;97(4):744-754.[20] 闫宇辉,李少恒,孔亮,等.NT-3高表达促进神经干细胞向胆碱能神经元分化[J].中国药理学通报,2016,32(5):631-637.[21] 魏洁,刘东方,王葱.中脑星形胶质细胞源性神经营养因子[J].中国生物化学与分子生物学报,2018,34(4):390-394. |