Chinese Journal of Tissue Engineering Research
Previous Articles Next Articles
Low-temperature synthesis and stabilization of carboxymethyl chitosan stabilized amorphous calcium phosphate
Wang Ping-ting, Qi Xing-ying
- Tianjin Medical University School & Hospital of Stomatology, Tianjin 300070, China
-
Received:2018-02-21Online:2018-08-08Published:2018-08-08 -
About author:Wang Ping-ting, Master, Attending physician, Tianjin Medical University School & Hospital of Stomatology, Tianjin 300070, China -
Supported by:the Natural Science Foundation of Tianjin, No. 16JCZDJC32800
CLC Number:
Cite this article
Wang Ping-ting, Qi Xing-ying . Low-temperature synthesis and stabilization of carboxymethyl chitosan stabilized amorphous calcium phosphate[J]. Chinese Journal of Tissue Engineering Research, doi: 10.3969/j.issn.2095-4344.0893.
share this article
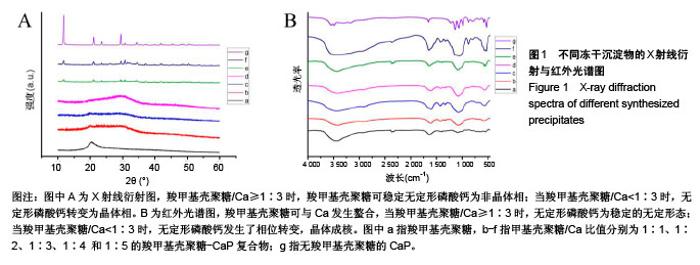
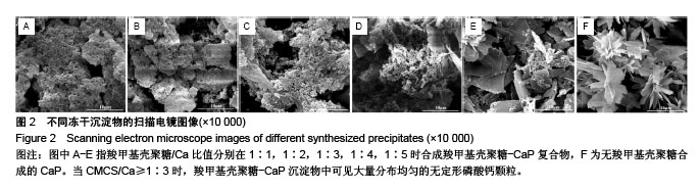
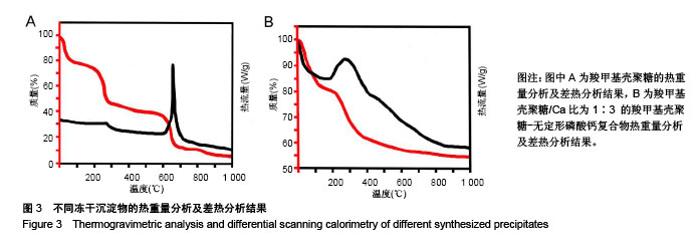
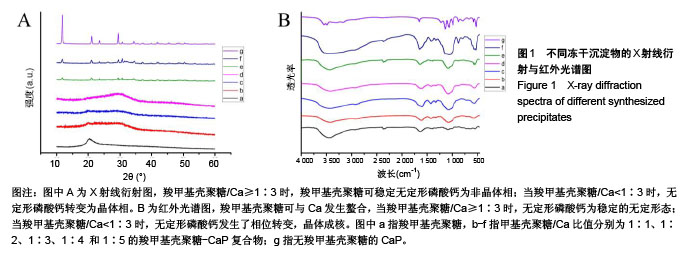
2.1 CMCS-无定形磷酸钙的结构与成分分析 6组冻干沉淀物及CMCS对照的X射线衍射图如图1A所示。CMCS对照组(a)可见2θ在20°附近存在CMCS的典型衍射峰,而在冻干沉淀物组中,随着CMCS/Ca比值降低,由1∶1组至1∶5组CMCS典型衍射峰逐渐降低。同时,1∶1组(b)、1∶2组(c)、1∶3组(d)中在30°左右可见非晶体衍射峰,而当CMCS/Ca比值降至1∶4(e)和1∶5(f)时出现了与无CMCS的CaP组(g)相似的晶体衍射峰。 6组冻干沉淀物及CMCS对照的红外光谱图如图1B所示,CMCS的伸缩振动峰在1 400 cm-1 和1 650 cm-1处(a),而在CMCS-CaP冻干沉淀物中这两个峰值均发生变化;在CMCS/Ca比值≥1∶3时,在550 cm-1(ν4 PO43-弯模)附近可见单一的光环峰(b-d);而当CMCS/Ca比值<1∶3或无CMCS时,该单一峰分裂为530 cm-1 和570 cm-1附近的两个峰(e-g)。 图2显示了6组冻干沉淀物在扫描电镜下的显微结构,无CMCS辅助合成的CaP呈薄片状晶体结构,片状晶体间无泥雪样物质存在(图2F);而CMCS-CaP冻干沉淀物各组均可见CaP以大量不规则形状的颗粒形式存在,颗粒间存在泥雪样物质(CMCS);CaP颗粒尺寸不随CMCS/Ca比值改变而发生可见变化,但随着CMCS/Ca比值降低,颗粒间的泥雪样物质逐渐减少至消失(图2A-E);当CMCS/Ca比值降低至1∶3时,CaP颗粒的轮廓变得模糊,同时可见少量晶体结构出现(图2C);当CMCS/Ca比值降低至1∶4时这一趋势更为明显(图2D);当CMCS/Ca比值进一步降低至1∶5时,可见大量晶体结构生成,不规则CaP颗粒及泥雪样物质显著减少(图2E)。 对于CMCS进行热重量及差热分析,在60 ℃附近观察到放热峰值,相应有19.17%的质量降低;加热到280 ℃时再次出现放热峰,同时重量也急剧减少(图3A);当温度升至1 000 ℃时剩余量降至为7.04%。显示了CMCS/Ca比为1∶3的CMCS-无定形磷酸钙复合物的热重量分析及差热分析结果,放热初期即有16.22%的质量丢失,放热峰值也降低到260 ℃附近;当加热到1000 ℃时CMCS-无定形磷酸钙复合物剩余量为53.31%(图3B)。根据以上提及的数据计算可得CMCS-无定形磷酸钙复合物中无定形磷酸钙的实际量为63.63%。"
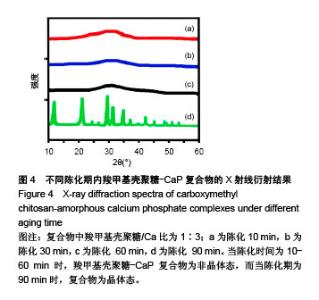
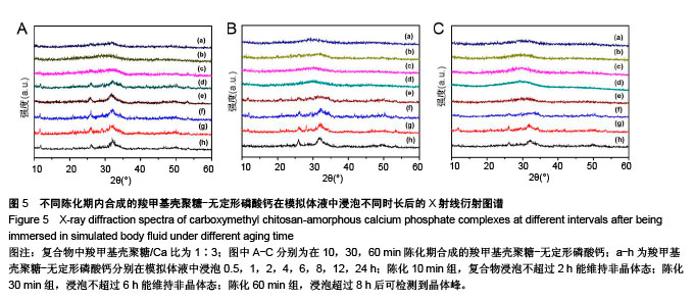
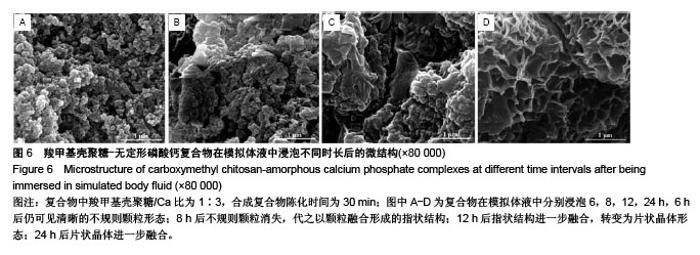
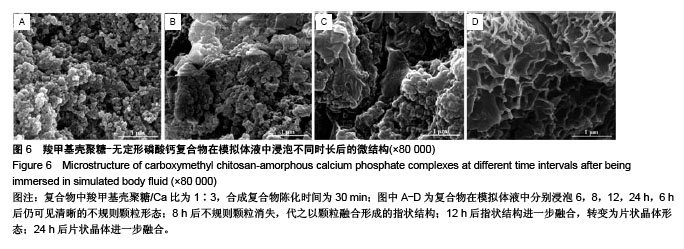
2.2 CMCS-无定形磷酸钙复合物相位转化分析 在CMCS/Ca比值为1∶3条件下,采用不同陈化时间制备的CMCS-CaP冻干沉淀物X射线衍射结果如图4所示,当陈化时间为10-60 min时产物为非晶体态,而当陈化期为90 min时复合物为晶体态。 陈化时间分别为10,30,60 min的3个实验组所得CMCS-无定形磷酸钙复合物的相位转化时间分别为2,6,8 h。各组复合物浸泡于模拟体液后测得的X射线衍射图谱见图5,陈化10 min组,复合物浸泡不超过2 h则能维持非晶体态;陈化30 min组,浸泡不超过6 h则能维持非晶体态;陈化60 min组,浸泡超过8 h后可检测到晶体峰。 图6显示了陈化30 min组CMCS-无定形磷酸钙复合物随浸泡时间延长而发生的显微结构变化:6 h后仍可见清晰的不规则颗粒形态;8 h后不规则颗粒消失,代之以颗粒融合形成的指状结构;12 h后指状结构进一步融合,转变为片状晶体形态;24 h后片状晶体进一步融合。另外两陈化时间组的CMCS-无定形磷酸钙复合物显微结构与陈化 30 min组类似。 "
| [1] Termine JD,Posner AS.Amorphous/crystalline interrelationships in bone mineral.Calcif Tissue Res. 1967;1(1):8-23.[2] Termine JD,Posner AS.Infrared absorption of carbonate apatite.Science.1967;155(3762): 607-608.[3] Vieira AEM,Danelon M,Camara DMD,et al.In vitro effect of amorphous calcium phosphate paste applied for extended periods of time on enamel remineralization.J Appl Oral Sci. 2017;25(6): 596-603. [4] Liu S,Weng W,Li Z,et al.Effect of PEG amount in amorphous calcium phosphate on its crystallized products.J Mater Sci Mater Med.2009;20(1):359-363.[5] Jin W,Jiang S,Pan H,et al.Amorphous Phase Mediated Crystallization: Fundamentals of Biomineralization. Crystals. 2018; 8(1):48.[6] Jiang S,Pan H,Chen Y,et al.Amorphous calcium phosphate phase-mediated crystal nucleation kinetics and pathway. Faraday Discuss.2015;179:451-461.[7] Li Y,Weng W.In vitro synthesis and characterization of amorphous calcium phosphates with various Ca/P atomic ratios.J Mater Sci Mater Med.2007;18(12):2303-2308.[8] Tadic D,Peters F,Epple M.Continuous synthesis of amorphous carbonated apatites. Biomaterials. 2002;23(12):2553-2559.[9] Nagano M,Nakamura T,Kokubo T,et al.Differences of bone bonding ability and degradation behaviour in vivo between amorphous calcium phosphate and highly crystalline hydroxyapatite coating.Biomaterials.1996;17(18):1771-1777.[10] Combes C,Rey C.Amorphous calcium phosphates: synthesis, properties and uses in biomaterials.Acta Biomater. 2010;6(9): 3362-3378.[11] Ma N,Ma C,Li C,et al.Influence of nanoparticle shape, size, and surface functionalization on cellular uptake.J Nanosci Nanotechnol. 2013;13(10):6485-6498.[12] Singh SS,Roy A,Lee B,et al. Study of hMSC proliferation and differentiation on Mg and Mg-Sr containing biphasic β-tricalcium phosphate and amorphous calcium phosphate ceramics.Mater Sci Eng C Mater Biol Appl.2016;64:219-228.[13] Par M,Šanti? A,Gamulin O,et al.Impedance changes during setting of amorphous calcium phosphate composites.Dent Mater. 2016;32(11):1312-1321.[14] Oyane A,Araki H,Nakamura M,et al.Controlled superficial assembly of DNA-amorphous calcium phosphate nanocomposite spheres for surface-mediated gene delivery.Colloids Surf B Biointerfaces. 2016;141:519-527.[15] Heravi F,Bagheri H,Rangrazi A,et al.Incorporation of CPP-ACP into Luting and Lining GIC: Influence on Wear Rate (in the Presence of Artificial Saliva) and Compressive Strength. Acs Biomater Sci Eng.2016;2(11):1867-1871.[16] Li Y,Wiliana T,Tam KC.Synthesis of amorphous calcium phosphate using various types of cyclodextrins.Mater Res Bull. 2007;42(5):820-827.[17] LuoJ,Qiu S,Zhou X,et al.In situ grafting polyethylene glycol chains onto amorphous calcium phosphate nanoparticles to improve the storage stability and organic solvent redispersibility. Colloids Surf A Physicochem Eng Asp. 2014;444(Supplement C): 81-88.[18] Delgado-López JM,Bertolotti F,Lyngsø J,et al.The synergic role of collagen and citrate in stabilizing amorphous calcium phosphate precursors with platy morphology. Acta Biomater.2017; 49: 555-562.[19] Li Y,Weng W,Cheng K,et al.Preparation of amorphous calcium phosphate in the presence of poly(ethylene glycol).J Mater Sci Lett.2003;22(14):1015-1016.[20] Chen Z,Cao S,Wang H,et al.Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries.PLoS One.2015; 10(1):e0116553.[21] Yang W,Fu J,Wang D,et al.Study on chitosan/polycaprolactone blending vascular scaffolds by electrospinning.J Biomed Nanotechnol.2010;6(3):254-259.[22] Wang T,Ji X,Jin L,et al.Fabrication and characterization of heparin-grafted poly-L-lactic acid-chitosan core-shell nanofibers scaffold for vascular gasket.ACS Appl Mater Interfaces. 2013; 5(9):3757-3763.[23] Fonseca-Santos B, Chorilli M.An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems.Mater Sci Eng C Mater Biol Appl. 2017;77: 1349-1362.[24] Zhu Y,Chen F.pH-responsive drug-delivery systems.Chem Asian J.2015;10(2):284-305.[25] Yin Y,Dang Q,Liu C,et al.Itaconic acid grafted carboxymethyl chitosan and its nanoparticles: Preparation, characterization and evaluation.Int J Biol Macromol.2017;102:10-18.[26] Lin YS,Renbutsu E,Morimoto M,et al.Preparation of stable chitosan-carboxymethyl dextran nanoparticles.J Nanosci Nanotechnol.2009;9(4):2558-2565.[27] Wang J,Zhang D,Liu F,et al.Structure and properties of chitosan derivatives modified calcium polyphosphate scaffolds.Polym Degrad Stab.2010;95(7):1205-1210.[28] Budiraharjo R,Neoh KG,Kang ET.Hydroxyapatite-coated carboxymethyl chitosan scaffolds for promoting osteoblast and stem cell differentiation.J Colloid Interface Sci. 2012;366(1): 224-232.[29] Ikawa N,Kimura T,Oumi Y,et al.Amino acid containing amorphous calcium phosphates and the rapid transformation into apatite.J Mater Chem.2009;19(28):4906-4913.[30] Niu X,Liu Z,Tian F,et al.Sustained delivery of calcium and orthophosphate ions from amorphous calcium phosphate and poly(L-lactic acid)-based electrospinning nanofibrous scaffold.Sci Rep. 2017;7:45655.[31] Bar-Yoaef O,Govrin-Lippman R,Garti N,et al.The Influence of Polyelectrolytes on the Formation and Phase Transformation of Amorphous Calcium Phosphate.Crystal Growth Design. 2004; 4(1):177-183.[32] Jin L,Zeng X,Liu M,et al.Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics.2014;4(3):240-255[2014]. https://www.ncbi.nlm.nih. gov/pmc/articles/PMC3915088/pdf/thnov04p0240.pdf.[33] Gupta RR,Kim H, Chan YK,et al.Axial strain enhances osteotomy repair with a concomitant increase in connexin43 expression. Bone Res.2015;3:15007[2015]. https://www.ncbi.nlm.nih. gov/pmc/ articles/PMC4411567/pdf/boneres20157.pdf.[34] Liu J,Schmidlin PR,Philipp A,et al.Novel bone substitute material in alveolar bone healing following tooth extraction: an experimental study in sheep.Clin Oral Implants Res. 2016;27(7): 762-770.[35] Azarpazhooh A,Limeback H.Clinical efficacy of casein derivatives: a systematic review of the literature.J Am Dent Assoc. 2008; 139(7):915-924;quiz 994-915.[36] Taranath A,Pai D,Pentapati K.The role of casein phosphopeptide-amorphous calcium phosphate products in remineralization of incipient enamel lesions and its substantivity.J Exp Integr Med.2014;4(1):67-70.[37] Huang LY,Liu TY,Liu KH,et al.Electrospinning of amphipathic chitosan nanofibers for surgical implants application.J Nanosci Nanotechnol.2012;12(6):5066-5070.[38] Sahariah P,Masson M.Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules.2017;18(11):3846-3868.[39] ChengBX,Pei BY,Wang ZK,et al.Advances in chitosan-based superabsorbent hydrogels. Rsc Adv.2017;7(67):42036-42046.[40] Asmatulu R,Patrick S,Ceylan M,et al.Antibacterial Polycaprolactone/Natural Hydroxyapatite Nanocomposite Fibers for Bone Scaffoldings.J Bionanosci.2016;9(2):1-7.[41] Huang GQ,Cheng LY,Xiao JX,et al.Preparation and characterization of O-carboxymethyl chitosan-sodium alginate polyelectrolyte complexes. Colloid Polym Sci. 2015;293(2):401-407.[42] Onuma K,Ito A.Cluster Growth Model for Hydroxyapatite. City, 1998.[43] Eanes E.Amorphous calcium phosphate.Monogr Oral Sci. 2001; 18:130-147.[44] Zhao J,Liu Y,Sun WB,et al.First detection, characterization, and application of amorphous calcium phosphate in dentistry.J Dent Sci.2012;7(4):316-323.[45] Zhao D,Huang J,Hu S,et al.Biochemical activities of N,O-carboxymethyl chitosan from squid cartilage.Carbohydr Polym.2011;85(4):832-837.[46] Wahid F,Wang HS,Zhong C,et al.Facile fabrication of moldable antibacterial carboxymethyl chitosan supramolecular hydrogels cross-linked by metal ions complexation.Carbohydr Polym. 2017;165:455-461.[47] Pan H,Liu XY,Tang R,et al.Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite.Chemical communications(Cambridge, England). 2010;46(39):7415-7417. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||