Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (16): 2593-2600.doi: 10.3969/j.issn.2095-4344.0805
Previous Articles Next Articles
Low-intensity pulsed ultrasound promotes bone and cartilage regeneration
Zu Hai-yue1, Yi Xue-ting2, Zhao De-wei1
- 1Department of Orthopedics, 2Department of Lithotripsy, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China
-
Received:2017-11-23Online:2018-06-08Published:2018-06-08 -
Contact:Zhao De-wei, Professor, Chief physician, Doctoral supervisor, Department of Orthopedics, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China -
About author:Zu Hai-yue, Studying for master’s degree, Department of Orthopedics, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China
CLC Number:
Cite this article
Zu Hai-yue1, Yi Xue-ting2, Zhao De-wei1. Low-intensity pulsed ultrasound promotes bone and cartilage regeneration[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(16): 2593-2600.
share this article
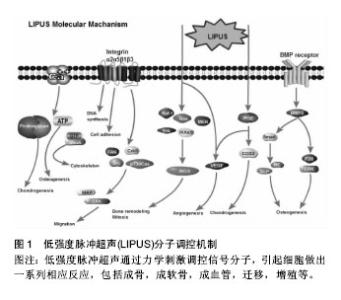
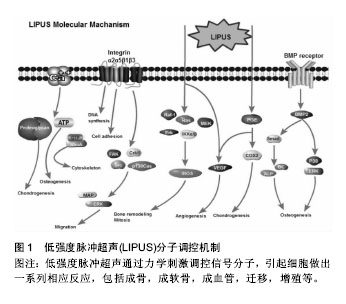
2.1 LIPUS调控细胞的分子机制 2.1.1 钙离子 钙离子是细胞力学信号通路中一种重要的生物学信号,其浓度变化是细胞对外界环境压力的反应之一。当钙离子通道被激活时,钙离子从细胞膜外或从细胞内存储的细胞器中(如内质网,肌质网和线粒体)转运入胞浆,引起了下游生物学变化。Parvizi等[6]研究发现,LIPUS可以增加软骨细胞内钙离子的浓度,使细胞内蛋白多糖含量上升。当利用钙离子螯合剂或Ca2+/ATP酶抑制剂后,发现LIPUS对细胞内钙离子影响显著减低,则证实LIPUS可以激活细胞内钙离子通道,或者也可以通过Ca2+和P2Y受体来调控ATP/嘌呤的释放,从而影响成骨细胞的增殖[7](图1)。钙离子在酶活动中起间接作用,其通过结合不同蛋白,包括钙调蛋白,肌钙蛋白和膜联蛋白完成变构,形成复合物来充当第二信使,并触发下游分子的调节[8]。最近的研究表明,LIPUS刺激人类骨髓间充质干细胞,可以调节其细胞内钙离子的摆动性[9]。钙离子的摆动性对于骨髓间充质干细胞分化等多种细胞功能至关重要。Kim等[10]研究发现,钙离子的摆动性通过影响RhoA的GTP酶通路来起作用,这些因子可作为细胞骨架调节的主要因素。然而,现阶段作者仍不能阐述LIPUS调节钙离子摆动性及促进钙离子内流的具体机制,在接下来的研究中需要进一步探讨。 "
| [1] Rubin C, Bolander M, Ryaby JP, et al. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am.2001;83-A(2): 259-270.[2] Moed BR, Kim EC,van Holsbeeck M,et al.Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: histologic correlation using a canine model. J Orthop Trauma.1998;12(3): 200-205.[3] Moed BR, Subramanian S,van Holsbeeck M, et al. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma.1998;12(3): 206-213.[4] Buchtala V. Present state of ultrasound therapy. Dia Med. 1950;22(70): 2944-2950.[5] Maintz G.Animal experiments in the study of the effect ofultrasonic waves on bone regeneration. Strahlentherapie.1950; 82(4): 631-638.[6] Parvizi J, Parpura V, Greenleaf JF, et al. Calcium signaling is required for ultrasound-stimulated aggrecan synthesis by rat chondrocytes. J Orthop Res.2002; 20(1): 51-57.[7] Alvarenga EC,Rodrigues R,Caricati-Neto A,et al.Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptor: role of the P2Y1 receptor. Bone.2010;46(2): 355-362.[8] Doroudi M,Plaisance MC,Boyan BD,et al.Membrane actions of 1alpha,25(OH)2D3 are mediated by Ca(2+)/calmodulin- dependent protein kinase II in bone and cartilage cells. J Steroid Biochem Mol Biol.2015;145: 65-74.[9] Sun S,Liu Y,Lipsky S,et al.Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J.2007;21(7):1472-1480.[10] Kim TJ, Seong J, Ouyang M, et al. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218(2): 285-293.[11] Tang CH, Yang RS, Huang TH, et al. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol. 2006; 69(6): 2047-2057.[12] Watabe H,Furuhama T,Tani-Ishii N,et al.Mechanotransduction activates alpha(5)beta(1) integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell Res.2011; 317(18): 2642-2649.[13] Zhou S, Schmelz A, Seufferlein T, et al. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts.J Biol Chem.2004;279(52): 54463-54469.[14] Sato M, Nagata K, Kuroda S, et al. Low-intensity pulsed ultrasound activates integrin-mediated mechanotransduction pathway in synovial cells. Ann Biomed Eng.2014;42(10): 2156-2163.[15] Angle SR,Sena K,Sumner DR, et al. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics. 2011; 51(3): 281-288.[16] Cheng K, Xia P, Lin Q, et al. Effects of low-intensity pulsed ultrasound on integrin-FAK-PI3K/Akt mechanochemical transduction in rabbit osteoarthritis chondrocytes. Ultrasound Med Biol.2014;40(7): 1609-1618.[17] Whitney NP,Lamb AC,Louw TM,et al.Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound Med Biol. 2012;38(10): 1734-1743.[18] Xia P, Shen S, Lin Q, et al. Low-Intensity Pulsed Ultrasound Treatment at an Early Osteoarthritis Stage Protects Rabbit Cartilage From Damage via the Integrin/Focal Adhesion Kinase/Mitogen-Activated Protein Kinase Signaling Pathway. J Ultrasound Med.2015; 34(11):1991-1999.[19] Saura M, Tarin C, Zaragoza C. Recent insights into the implication of nitric oxide in osteoblast differentiation and proliferation during bone development. Scientific World J. 2010;10: 624-632.[20] Wang FS,Kuo YR,Wang CJ,et al.Nitric oxide mediates ultrasound-induced hypoxia-inducible factor-1alpha activation and vascular endothelial growth factor-A expression in human osteoblasts. Bone.2004;35(1):114-123.[21] Tang CH, Lu DY, Tan TW, et al. Ultrasound induces hypoxia-inducible factor-1 activation and inducible nitric-oxide synthase expression through the integrin/integrin-linked kinase/Akt/mammalian target of rapamycin pathway in osteoblasts. J Biol Chem.2007;282(35): 25406-25415.[22] Hou CH, Lin J, Huang SC, et al. Ultrasound stimulates NF-kappaB activation and iNOS expression via the Ras/Raf/MEK/ERK signaling pathway in cultured preosteoblasts. J Cell Physiol. 2009;220(1): 196-203.[23] Herrera BS, Martins-Porto R, Maia-Dantas A, et al. iNOS-derived nitric oxide stimulates osteoclast activity and alveolar bone loss in ligature-induced periodontitis in rats. J Periodontol.2011;82(11): 1608-1615.[24] Jung DH, Kim KH, Byeon HE, et al. Involvement of ATF3 in the negative regulation of iNOS expression and NO production in activated macrophages. Immunol Res.2015; 62(1): 35-45.[25] Wang G, Yan Q, Woods A, et al. Inducible nitric oxide synthase-nitric oxide signaling mediates the mitogenic activity of Rac1 during endochondral bone growth. J Cell Sci.2011; 124(Pt 20): 3405-3413.[26] Kawaguchi H,Pilbeam CC,Harrison JR,et al.The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res.1995;(313): 36-46.[27] Kokubu T, Matsui N, Fujioka H, et al. Low intensity pulsed ultrasound exposure increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in mouse osteoblasts. Biochem Biophys Res Commun.1999;256(2): 284-287.[28] Reher P, Harris M, Whiteman M, et al. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone.2002;31(1): 236-241.[29] Rego EB, Inubushi T, Kawazoe A, et al. Ultrasound stimulation induces PGE(2) synthesis promoting cementoblastic differentiation through EP2/EP4 receptor pathway. Ultrasound Med Biol.2010;36(6): 907-915.[30] Naruse K, Sekiya H, Harada Y, et al. Prolonged endochondral bone healing in senescence is shortened by low-intensity pulsed ultrasound in a manner dependent on COX-2. Ultrasound Med Biol.2010;36(7):1098-1108.[31] Hidaka K, Miyamoto C, Kawata A, et al.13. Effect of Low-Intensity Pulsed Ultrasound (LIPUS) on Remote Bone Marrow in Rats With Healing Socket. J Orthop Trauma, 2016; 30(8): S5-6.[32] Saini V, Yadav S, McCormick S. Low-intensity pulsed ultrasound modulates shear stress induced PGHS-2 expression and PGE2 synthesis in MLO-Y4 osteocyte-like cells. Ann Biomed Eng.2011;39(1): 378-393.[33] Murray SS, Brochmann Murray EJ, Wang JC, et al. The history and histology of bone morphogenetic protein. Histol Histopathol.2016;31(7): 721-732.[34] Ruschke K, Hiepen C, Becker J, et al. BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res.2012;347(3):521-544.[35] Suzuki A, Takayama T, Suzuki N, et al. Daily low-intensity pulsed ultrasound stimulates production of bone morphogenetic protein in ROS 17/2.8 cells. J Oral Sci.2009; 51(1): 29-36.[36] Shen B, Wei A, Whittaker S, et al. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem.2010;109(2): 406-416.[37] Hernandez-Hurtado AA, Borrego-Soto G, Marino-Martinez IA, et al. Implant Composed of Demineralized Bone and Mesenchymal Stem Cells Genetically Modified with AdBMP2/AdBMP7 for the Regeneration of Bone Fractures in Ovis aries. Stem Cells Int. 2016;2016: 7403890.[38] Huang W, Hasegawa T, Imai Y, et al. Low-intensity pulsed ultrasound enhances bone morphogenetic protein expression of human mandibular fracture haematoma-derived cells. Int J Oral Maxillofac Surg.2015;44(7): 929-935.[39] Lai CH, Chen SC, Chiu LH, et al. Effects of low-intensity pulsed ultrasound, dexamethasone/TGF-beta1 and/or BMP-2 on the transcriptional expression of genes in human mesenchymal stem cells: chondrogenic vs. osteogenic differentiation. Ultrasound Med Biol.2010;36(6): 1022-1033.[40] Lee SY,Koh A,Niikura T,et al.Low-intensity pulsed ultrasound enhances BMP-7-induced osteogenic differentiation of human fracture hematoma-derived progenitor cells in vitro. J Orthop Trauma.2013;27(1): 29-33.[41] Naruse K, Miyauchi A, Itoman M, et al. Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J Bone Miner Res.2003;18(2): 360-369.[42] Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem.2005; 280(36): 31353-31359.[43] Sena K, Leven RM, Mazhar K, et al. Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells. Ultrasound Med Biol.2005;31(5):703-708.[44] Bozec A, Bakiri L, Jimenez M, et al. Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production. J Cell Biol.2010;190(6):1093-1106.[45] Gleizal A, Li S, Pialat JB, et al. Transcriptional expression of calvarial bone after treatment with low-intensity ultrasound: an in vitro study. Ultrasound Med Biol.2006;32(10): 1569-1574.[46] Yang RS, Lin WL, Chen YZ, et al. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone.2005;36(2):276-283.[47] Ting SY, Montagne K, Nishimura Y,et al. Modulation of the Effect of Transforming Growth Factor-beta3 by Low-Intensity Pulsed Ultrasound on Scaffold-Free Dedifferentiated Articular Bovine Chondrocyte Tissues. Tissue Eng Part C Methods. 2015; 21(10):1005-1014.[48] Guha Thakurta S,Budhiraja G,Subramanian A.Growth factor and ultrasound-assisted bioreactor synergism for human mesenchymal stem cell chondrogenesis. J Tissue Eng.2015;6: 2041731414566529.[49] Shafaei H, Esfandiari E, Esmaeili A, et al. Optimizing a novel method for low intensity ultrasound in chondrogenesis induction. Adv Biomed Res.2013;2:79.[50] Kobayashi Y,Sakai D,Iwashina T,et al.Low-intensity pulsed ultrasound stimulates cell proliferation, proteoglycan synthesis and expression of growth factor-related genes in human nucleus pulposus cell line. Eur Cell Mater.2009;17: 15-22.[51] Subramanian A, Turner JA, Budhiraja G, et al. Ultrasonic bioreactor as a platform for studying cellular response. Tissue Eng Part C Methods.2013;19(3): 244-255.[52] Jia L, Chen J, Wang Y, et al. Focused Low-intensity Pulsed Ultrasound Affects Extracellular Matrix Degradation via Decreasing Chondrocyte Apoptosis and Inflammatory Mediators in a Surgically Induced Osteoarthritic Rabbit Model. Ultrasound Med Biol.2016;42(1): 208-219.[53] 杜登悝,易刚,唐赢. 低强度脉冲超声对人骨性关节炎软骨细胞的影响[J]. 第三军医大学学报, 2016,38(21): 2320-2325.[54] 徐守宇,张丽梅,姚新苗. 低强度脉冲超声对兔膝骨关节炎软骨细胞外基质的影响及机制[J]. 中国骨伤, 2014,27(9): 766-771.[55] 刘洋,刘宁,刘昭铭. 低强度脉冲式超声对关节软骨的修复[J].中国组织工程研究, 2016,20(29): 4284-4289.[56] Uchida K, Urabe K, Naruse K, et al. 5. Accelerated Fracture Healing Targeting Periosteal Cells: Possibility of Combined Therapy of Low-Intensity Pulsed Ultrasound (LIPUS), Bone Graft, and Growth Factor (bFGF). J Orthop Trauma.2016; 30(8): S3.[57] Bernardi M, Albiero E, Alghisi A, et al. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy.2013;15(8): 920-929.[58] Nolte P, Anderson R, Strauss E, et al. Heal rate of metatarsal fractures: A propensity-matching study of patients treated with low-intensity pulsed ultrasound (LIPUS) vs. surgical and other treatments. Injury.2016;47(11): 2584-2590.[59] Kinami Y, Noda T, Ozaki T. Efficacy of low-intensity pulsed ultrasound treatment for surgically managed fresh diaphyseal fractures of the lower extremity: multi-center retrospective cohort study. J Orthop Sci.2013;18(3): 410-418.[60] Hannemann PF, Mommers EH, Schots JP, et al. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg.2014;134(8): 1093-1106.[61] Watanabe Y, Arai Y, Takenaka N, et al.Three key factors affecting treatment results of low-intensity pulsed ultrasound for delayed unions and nonunions: instability, gap size, and atrophic nonunion. J Orthop Sci.2013;18(5): 803-810.[62] Salem KH, Schmelz A. Low-intensity pulsed ultrasound shortens the treatment time in tibial distraction osteogenesis. Int Orthop.2014;38(7):1477-1482.[63] Dudda M, Hauser J, Muhr G, et al. Low-intensity pulsed ultrasound as a useful adjuvant during distraction osteogenesis: a prospective, randomized controlled trial. J Trauma.2011;71(5):1376-1380.[64] Tsumaki N,Kakiuchi M,Sasaki J,et al.Low-intensity pulsed ultrasound accelerates maturation of callus in patients treated with opening-wedge high tibial osteotomy by hemicallotasis. J Bone Joint Surg Am.2004; 86-A(11): 2399-2405.[65] Urita A, Iwasaki N, Kondo M, et al. Effect of low-intensity pulsed ultrasound on bone healing at osteotomy sites after forearm bone shortening. J Hand Surg Am. 2013;38(3): 498-503.[66] Jia L,Wang Y, Chen J, et al. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci Rep.2016;6: 35453.[67] Loyola-Sanchez A, Richardson J, Beattie KA, et al. Effect of low-intensity pulsed ultrasound on the cartilage repair in people with mild to moderate knee osteoarthritis: a double-blinded, randomized, placebo-controlled pilot study. Arch Phys Med Rehabil.2012;93(1): 35-42.[68] Zhou X, Castro NJ, Zhu W, et al. Improved Human Bone Marrow Mesenchymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci Rep.2016; 6: 32876.[69] Nagasaki R, Mukudai Y, Yoshizawa Y, et al. A Combination of Low-Intensity Pulsed Ultrasound and Nanohydroxyapatite Concordantly Enhances Osteogenesis of Adipose-Derived Stem Cells From Buccal Fat Pad. Cell Med.2015;7(3): 123-131.[70] 郭杨,马勇,董睿.低强度脉冲超声对同种异体软骨细胞-藻酸钙凝胶复合物修复兔膝关节软骨缺损的影响[J].中国修复重建外科杂志, 2013,27(8): 928-934.[71] 刘青,梁建飞.低强度的脉冲超声对钛种植体骨结合的影响[J].中国超声医学杂志, 2015,31(2):164-166.[72] 秦丽梅,吴琳. 低强度脉冲超声波作用下羟基磷灰石/磷酸三钙三维支架上成骨细胞的生物学行为的研究[J]. 生物医学工程与临床, 2014,18(6): 517-522.[73] Poolman RW, Agoritsas T, Siemieniuk RA, et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ.2017;356:j576. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||