Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (1): 139-143.doi: 10.3969/j.issn.2095-4344.0527
Previous Articles Next Articles
Paracrine actions of bone marrow mesenchymal stem cells: angiogenesis, immunoregulation, and inflammatory regulation
Zhang Xin, Xie Jiabing
- Department of Orthopedics Trauma, Yijishan Hospital, Wannan Medical College, Wuhu 241001, Anhui Province, China
-
Revised:2018-04-18Online:2019-01-08Published:2018-11-28 -
About author:Zhang Xin, Master, Physician, Department of Orthopedics Trauma, Yijishan Hospital, Wannan Medical College, Wuhu 241001, Anhui Province, China -
Supported by:the Scientific Research Foundation for the Middle-Young Age in Wannan Medical College, No. WK2014F14 (to ZX)
CLC Number:
Cite this article
Zhang Xin, Xie Jiabing. Paracrine actions of bone marrow mesenchymal stem cells: angiogenesis, immunoregulation, and inflammatory regulation[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(1): 139-143.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
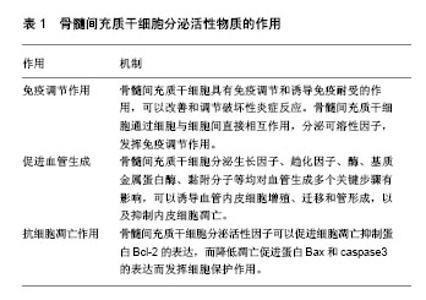
2.1 骨髓间充质干细胞在实验室体外环境下增殖及培养 目前在实验室体外环境下对骨髓间充质干细胞进行培养及分离的方法主要有全骨髓贴壁分离法、密度梯度离心法、流式细胞仪分离法和免疫磁珠分离法。后两种方法主要是应用骨髓间充质干细胞表面特殊抗原,对其经行筛选、分离、洗涤等步骤最终起到纯化的作用,但目前仍未找到骨髓间充质干细胞最具特异性的细胞表面标记,且实验成本较高,操作步骤繁琐,技术难度高。密度梯度离心法需要时间较长,且操作相对复杂。全骨髓贴壁分离法则相对简单,对新鲜的骨髓全血进行初步的离心,分离出所需要的底层细胞经行培养,利用骨髓间充质干细胞贴壁生长的特性,通过多次更换培养液,去除悬浮细胞,最终起到纯化的目的。 骨髓间充质干细胞的形态以梭形为主,少数呈三角形、星形,细胞传代后快速增殖,呈漩涡状排列,透射电镜下细胞形态不一,多为圆形,核仁清晰,核浆比例大,胞质中含有核糖体、粗面内质网、分泌小泡、线粒体等,细胞表面有微绒毛突起。缝隙连接可加强相邻细胞间的连接,有利于细胞间信息传递[3-4]。 ISCT已提出了一系列的标准来鉴定间充质干细胞:①在标准培养条件下,细胞能够贴壁生长,并呈成纤维样形态。事实上,一些学者认为,骨髓间充质干细胞和成纤维细胞在功能上是相同的[5];②细胞表面表达一些特异性抗原(标记物),骨髓间充质干细胞表面有CD73,CD90和CD105表面标志物表达,而缺乏CD11b,CD14,CD19,CD34,CD45,CD79a和HLA-DR表面标志物的表达[6];③具有向脂肪细胞、成骨细胞、软骨细胞分化的能力。 2.2 骨髓间充质干细胞分泌的活性物质 骨髓原始间充质干细胞不仅仅能够为骨髓中的造血干细胞提供物理性附着场所,而且本身还能分泌出多种有利于造血干细胞分化的生长因子,如白细胞介素6、白细胞介素11、白血病抑制因子、巨噬细胞集落刺激因子及干细胞因子等。 既往多项研究发现,骨髓间充质干细胞培养上清可能是通过生长因子、细胞因子等旁分泌效应对损伤组织起修复作用,通过动物实验发现,发挥损伤修复作用的物质可能是一种直径在50-100 nm的磷脂膜包裹的小囊泡,后被证实为骨髓间充质干细胞来源的微泡,它是骨髓间充质干细胞在静息或应激状态下产生的异质性膜分泌体系,由脂质双分子膜包裹,内含脂质、蛋白质、mRNA、miRNA等多种生物活性物质。 骨髓间充质干细胞是一类低免疫原性细胞,可调控树突状细胞和T细胞的功能,具有免疫调节和诱导免疫耐受的作用[7-9],可以改善和调节破坏性炎症反应。已知骨髓间充质干细胞通过细胞与细胞间直接相互作用,分泌可溶性因子,抑制T、B细胞的增殖,抑制中性粒细胞产生H2O2,抑制T细胞和NK细胞的细胞毒作用,以及抑制单核细胞的分化和成熟。 2.3 骨髓间充质干细胞分泌活性物质的作用 骨髓间充质干细胞有很强的自我扩增能力,同时能够保持多向分化性能,其能多向分化为成骨细胞、软骨细胞、脂肪细胞、心肌细胞、神经元细胞等。在体内,骨髓间充质干细胞受到各种营养机制激活时能够向多种组织类型的细胞分化,当接触到损伤环境时再生潜力提高。组织损伤发生后,内源性骨髓间充质干细胞感知损伤信号移出骨髓,进入外周血循环向损伤组织迁移,与靶组织血管内皮细胞黏附,并穿过胞外基质屏障,然后到达损伤组织进行修复[10-12]。 缺氧是导致细胞和组织损伤最常见的原因。早期炎性反应可以激活凝血系统,启动凝血级联反应,同时生理性纤溶途径被抑制,导致微循环障碍,组织缺血缺氧,出现器官功能障碍。缺氧诱导因子1是缺氧应答中起重要作用的转录因子,它通过控制血管生成来调节氧的运输能力,通过调控糖酵解作用来增强机体对低氧的免疫耐受。 多项研究表明,将骨髓间充质干细胞暴露于缺氧条件培养,以模仿损伤后的体内缺血性环境,可上调促生存和血管生成因子表达。低氧预处理骨髓间充质干细胞,可提高血管生成因子的含量,有助于减轻损伤和改善功能修复,提高治疗效果。骨髓间充质干细胞在缺氧环境中暴露24 h,能够分泌成纤维细胞生长因子2、肝细胞生长因子、胰岛素样生长因子1,可能是通过NF-κB信号通路所介导的[13-14]。骨髓间充质干细胞分泌的血管内皮生长因子水平进行性增高。低氧可以上调骨髓间充质干细胞表面的SDF-1受体趋化因子受体CXCR4的表达,促进骨髓间充质干细胞的迁移;低氧还促进骨髓间充质干细胞的增殖,发挥再生潜力[15]。骨髓间充质干细胞在体外可以显著抑制T淋巴细胞增殖,通过分泌免疫抑制性细胞因子以及表达IDO等途径构建局部免疫微环境,发挥免疫抑制作用,在γ-干扰素作用下,骨髓间充质干细胞免疫抑制活性增强[16-20]。炎症刺激条件下,骨髓间充质干细胞通过分泌转化生长因子β、肝细胞生长因子等多种蛋白因子[21],可以抑制细胞或体液免疫中的T淋巴细胞或B淋巴细胞增殖,并能将已被激活的T淋巴细胞或B淋巴细胞诱导失活。因此,骨髓间充质干细胞可以在炎症组织内发挥免疫调节的作用,该作用在组织修复中具有极为广阔的应用前景[22]。 肿瘤坏死因子也是一种炎性介质,可以诱导炎症,诱发组织坏死。肿瘤坏死因子α预处理的骨髓间充质干细胞比未经处理的骨髓间充质干细胞具有更优的体外血管生成活性[23]。另一项研究表明,肿瘤坏死因子α预处理通过上调骨形态发生蛋白2蛋白水平增加细胞增殖、动员和成骨分化。骨形态发生蛋白2基因沉默后抑制了骨髓间充质干细胞诱导成骨分化[24]。最近的研究表明,先天免疫激活剂,如脂多糖、Toll样受体激动剂,也可通过旁分泌因子刺激骨髓间充质干细胞增殖活性[25]。 炎症介质干扰素、肿瘤坏死因子可通过细胞外基质调节骨髓间充质干细胞归巢和迁移[26],在体外,骨髓间充质干细胞表现出对各种伤口愈合细胞因子有趋化作用[26-27]。将小鼠和人骨髓间充质干细胞植入到炎症和组织损伤区域[28-30],可促进伤口愈合、骨组织再生,调节免疫反应[31]。骨髓间充质干细胞可以抑制肥大细胞[32]、T细胞[33]、B细胞和自然杀伤细胞的募集[34-35],从而减轻损伤。伤口的炎症环境也激发了骨髓间充质干细胞内COX-2的活性,上调前列腺素E2表达[36],有利于骨组织的再生。前列腺素E2还可以使白细胞介素2、γ-干扰素表达减少,白细胞介素4和白细胞介素10表达增加[37-40]。抗炎细胞因子的表达有利于成纤维细胞基质金属蛋白酶表达上调和胶原蛋白表达下调[41-42],从而在创伤部位形成密度较小、纤维化的肉芽组织。骨髓间充质干细胞在损伤部位的活性超过炎症反应,其分化为成骨细胞促进骨组织的再生[42-43]。此外,还有大量的证据表明,骨髓间充质干细胞在增殖阶段继续支持组织再生。在此阶段,血管生成是必需的,以确保有足够的营养输送到损伤部位,加快伤口愈合。损伤部位微血管不足可能会抑制伤口愈合和导致慢性伤口未愈合。骨髓间充质干细胞可以分泌出多种细胞因子,包括血管内皮生长因子A、肾上腺髓质素、碱性成纤维细胞生长因子[44],这些因子可以促进微血管内皮细胞增殖[45-48],形成一个长期持久的功能性血管网络[49]。骨髓间充质干细胞还可以表达其他的多种细胞生长因子,包括白细胞介素10、肝细胞生长因子。 骨髓间充质干细胞分泌活性物质的相关作用见表1。"

| [1] Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664-668.[2] Yang CC, Shih YH, Ko MH, et al. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008; 3(10):e3336.[3] Kadner A, Hoerstrup SP, Zund G, et al. A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur J Cardiothorac Surg. 2002;21(6):1055-1060.[4] Brighton CT, Hunt RM. Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg Am. 1991;73(6):832-847.[5] Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity. Cytotherapy. 2012;14(5):516-521.[6] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.[7] Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8.[8] Ryan JM, Barry F, Murphy JM, et al. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007; 149(2):353-363.[9] Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25 (11):2896-2902.[10] Caplan AI. All MSCs are pericytes. Cell Stem Cell. 2008;3(3): 229-230.[11] Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318-324.[12] Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.[13] Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294(3):C675-682. [14] Rasmussen JG, Frøbert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011; 13(3):318-328.[15] Efimenko A, Starostina E, Kalinina N, et al. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10.[16] Rong LJ, Chi Y, Yang SG, et al. Effects of interferon-γ on biological characteristics and immunomodulatory property of human umbilical cord-derived mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20(2):421-426.[17] Kang JW, Koo HC, Hwang SY, et al. Immunomodulatory effects of human amniotic membrane-derived mesenchymal stem cells. J Vet Sci. 2012;13(1):23-31.[18] Lin W, Oh SK, Choo AB, et al. Activated T cells modulate immunosuppression by embryonic-and bone marrow-derived mesenchymal stromal cells through a feedback mechanism. Cytotherapy. 2012;14(3):274-284.[19] Croitoru-Lamoury J, Lamoury FM, Caristo M, et al. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One. 2011;6(2):e14698.[20] Tu Z, Li Q, Bu H, et al. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19(11):1803-1809.[21] Ryan JM, Barry F, Murphy JM, et al. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells.Clin Exp Immunol.2007;149(2): 353-363.[22] Noone C, Kihm A, English K, et al. IFN-γ stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013;22(22):3003-3014. [23] Kwon YW, Heo SC, Jeong GO, et al. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832(12):2136-2144.[24] Lu Z, Wang G, Dunstan CR, et al. Activation and promotion of adipose stem cells by tumour necrosis factor-α preconditioning for bone regeneration. J Cell Physiol. 2013;228(8):1737-1744.[25] Grote K, Petri M, Liu C, et al. Toll-like receptor 2/6-dependent stimulation of mesenchymal stem cells promotes angiogenesis by paracrine factors. Eur Cell Mater. 2013;26:66-79.[26] Hemeda H, Jakob M, Ludwig AK, et al. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19(5):693-706.[27] Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26(10): 1407-1412.[28] Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407-8411.[29] Chen L, Tredget EE, Liu C, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4(9):e7119.[30] Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737-1745.[31] Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2): 141-150.[32] Brown JM, Nemeth K, Kushnir-Sukhov NM, et al. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy. 2011;41(4):526-534.[33] Djouad F, Bouffi C, Ghannam S, et al. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5(7):392-399.[34] Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1): 367-372.[35] Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74-85.[36] Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42-49.[37] Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8): 2025-2032.[38] Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181(6):4389-4396.[39] Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4): 1815-1822.[40] Benbernou N, Esnault S, Shin HC, et al. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997;91(3):361-368.[41] Reitamo S, Remitz A, Tamai K, et al. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94(6): 2489-2492.[42] Jeon YK, Jang YH, Yoo DR, et al. Mesenchymal stem cells' interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010;18(6):655-661.[43] Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128(7):1852-1860.[44] Renault MA, Roncalli J, Tongers J, et al. The Hedgehog transcription factor Gli3 modulates angiogenesis. Circ Res. 2009;105(8):818-826.[45] Gruber R, Kandler B, Holzmann P, et al. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11(5-6):896-903.[46] Kaigler D, Krebsbach PH, Polverini PJ, et al. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9(1):95-103.[47] Lozito TP, Taboas JM, Kuo CK, et al. Mesenchymal stem cell modification of endothelial matrix regulates their vascular differentiation. J Cell Biochem. 2009;107(4):706-713.[48] Kato J, Tsuruda T, Kita T, et al. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005; 25(12):2480-2487.[49] Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551-4558. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [14] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [15] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||