Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5167-5177.doi: 10.12307/2026.331
Previous Articles Next Articles
Construction and osteogenic activity of titanium dioxide nanotube and polydopamine composite coating on titanium implants
Sun Danhe1, Guo Xiaoling2, Zhao Lingzhou3
- 1School of Life Sciences, Northwest University, Xi’an 710069, Shaanxi Province, China; 2Fifth Clinical Medical College, Anhui Medical University, Hefei 230032, Anhui Province, China; 3Department of Stomatology, Air Force Medical Center, Beijing 100142, China
-
Accepted:2025-04-03Online:2026-07-18Published:2025-11-24 -
Contact:Zhao Lingzhou, PhD, Associate chief physician, Master’s supervisor, Department of Stomatology, Air Force Medical Center, Beijing 100142, China -
About author:Sun Danhe, Master candidate, School of Life Sciences, Northwest University, Xi’an 710069, Shaanxi Province, China -
Supported by:Rapid Response Research Fund Project of Air Force Medical Center, No. 2024ZXKXKT012 (to ZLZ)
CLC Number:
Cite this article
Sun Danhe, Guo Xiaoling, Zhao Lingzhou. Construction and osteogenic activity of titanium dioxide nanotube and polydopamine composite coating on titanium implants[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5167-5177.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

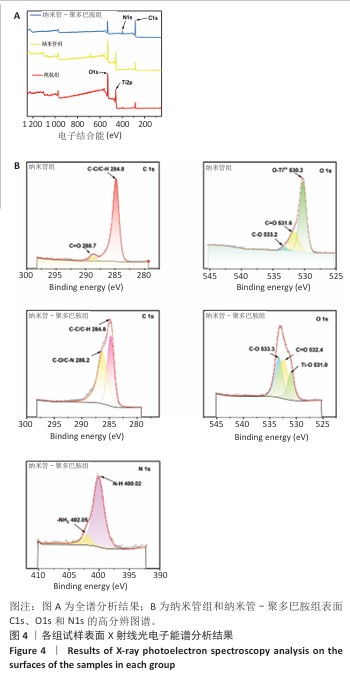
2.4 各组试样X射线光电子能谱分析结果 通过X射线光电子能谱对纳米管-聚多巴胺复合涂层的多级结合机制进行系统性解析,揭示界面键合的本质特征及涂层稳定化原理。图4A全谱分析所示,聚多巴胺特征性N1s峰的出现及钛基底信号(Ti2p)的完全屏蔽,证实聚多巴胺涂层实现了对纳米管阵列的致密包覆,这种全覆盖特性源于聚多巴胺分子在纳米管表面的多重结合模式:高分辨C1s谱(图4B)显示286 eV处C-O键强度显著提升,同时O=C键结合能发生0.8 eV正移,这共同指向酚羟基通过氧化偶联反应与基底形成化学锚定。N1s谱(图4B)中402.05 eV(-NH2)与 400.02 eV(-N-H)的双峰结构,结合O1s谱(图4B)中O=C键比例提升,证实邻苯二酚基团在碱性环境下的氧化自聚过程——醌式结构的生成触发了迈克尔加成(C-N键形成)和席夫碱反应(C=N键构建),最终形成三维交联网络。这种化学交联与聚多巴胺分子链的物理缠绕协同作用,在纳米管表面构筑出具有机械互锁效应的复合界面;此外,氢键作用进一步强化了涂层与基底结合强度,形成多尺度协同的稳定化体系。该复合结合机制不仅赋予涂层优异的界面稳定性,表面丰富的氨基和酚羟基更为生物活性分子的定向固定提供了多功能位点,为构建钛基生物材料的仿生功能界面奠定了科学基础。 "
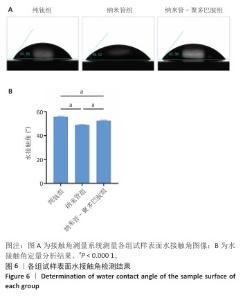
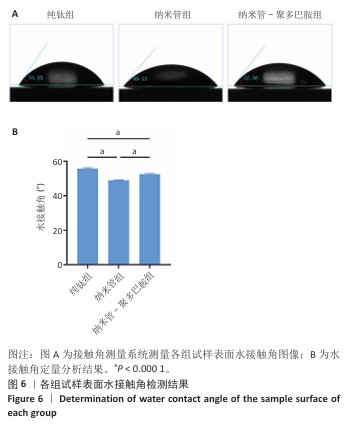
2.6 各组试样表面亲水性检测结果 采用接触角测量系统对各组试样表面的亲水性进行表征,结果如图6所示。纯钛组、纳米管组和纳米管-聚多巴胺组试样表面水接触角分别为(55.70±0.55)°,(49.02±0.19)°和(52.55±0.45)°。与纯钛组相比,纳米管组水接触角显著降低6.68°(P < 0.000 1),纳米管组试样表面亲水性提升主要源于两方面协同作用:①阳极氧化形成的纳米管阵列通过毛细效应增强液体渗透;②表面富集的Ti-OH极性基团显著提高材料表面能[33]。值得注意的是,纳米管-聚多巴胺组试样表现水接触角较纳米管组增加3.53°(P < 0.000 1),但仍比纯钛组低3.15°(P < 0.000 1),这是由于聚多巴胺分子中氨基/邻苯二酚等亲水基团与羰基的疏水特性形成动态平衡,这种两亲性特征使它的亲水性介于纯无机表面与有机涂层之间。适度的表面亲水性可通过调控纤维蛋白吸附构象,促进成骨细胞的黏着斑形成及定向分化,这对骨结合过程具有重要促进作用。 "
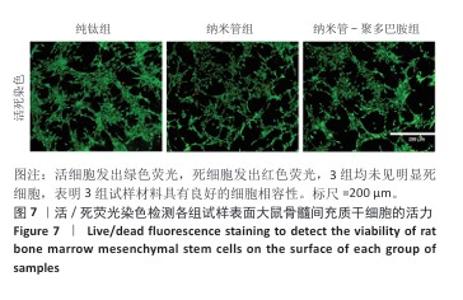
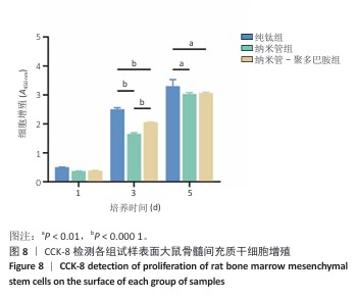
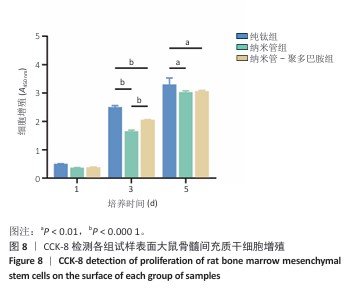
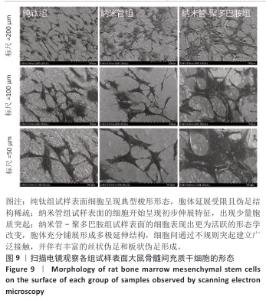
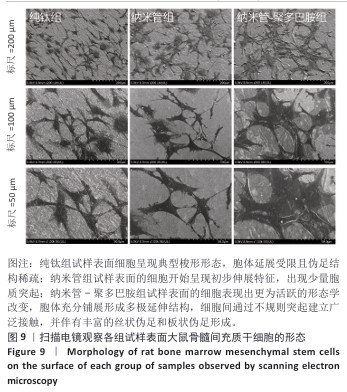
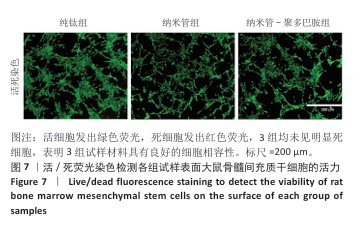
2.7 各组试样表面大鼠骨髓间充质干细胞的增殖与活性检测结果 活/死荧光染色结果显示,纯钛组、纳米管组及纳米管-聚多巴胺组表面培养的大鼠骨髓间充质干细胞均未发生明显死亡现象,见图7,显示出良好的细胞相容性。CCK-8检测结果显示,随着培养时间的延长,3组试样表面的大鼠骨髓间充质干细胞持续增殖,培养1 d,各组细胞增殖比较差异无显著性意义(P > 0.05);培养3 d,纳米管组与纳米管-聚多巴胺组细胞增殖均低于纯钛组(P < 0.000 1),纳米管-聚多巴胺组细胞增殖高于纳米管组(P < 0.000 1);培养5 d,纳米管组与纳米管-聚多巴胺组细胞增殖低于纯钛组(P < 0.01),见图8。纳米管组与纳米管-聚多巴胺组细胞增殖活性相对降低的现象,可能与材料表面拓扑结构诱导的细胞分化启动有关。"
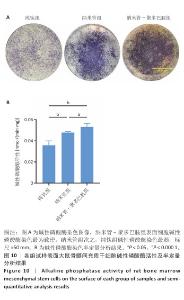
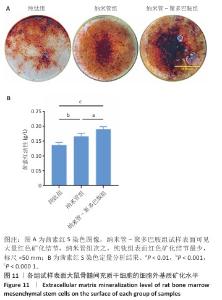
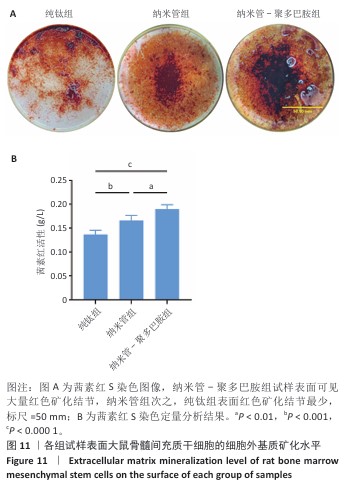
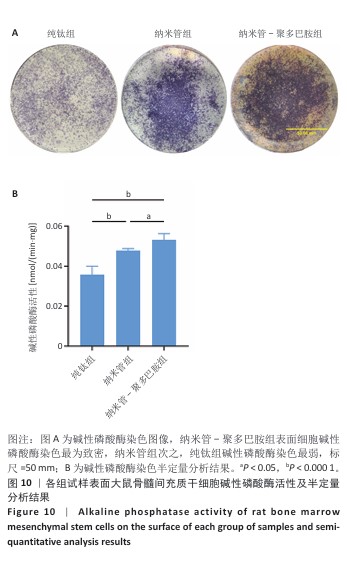
2.9 各组试样表面大鼠骨髓间充质干细胞成骨分化检测结果 图10显示成骨诱导7 d后各组试样表面大鼠骨髓间充质干细胞的碱性磷酸酶染色及半定量分析结果。纳米管-聚多巴胺组呈现出最致密的碱性磷酸酶染色,纳米管组次之,纯钛组染色强度相对最弱。半定量分析结果显示,纳米管组、纳米管-聚多巴胺组碱性磷酸酶活性高于纯钛组(P < 0.000 1),纳米管-聚多巴胺组碱性磷酸酶活性高于纳米管组(P < 0.05)。上述结果表明,纳米管-聚多巴胺复合涂层的纳米形貌与化学修饰具有协同增效作用,可显著增强大鼠骨髓间充质干细胞的成骨分化潜能。 图11展示成骨诱导培养14 d后各组试样表面大鼠骨髓间充质干细胞的茜素红S染色及定量分析结果。纳米管-聚多巴胺组呈现出最深且覆盖面积最大的染色区域,纳米管组次之,纯钛组染色区域最为浅淡。定量分析结果显示,纳米管组、纳米管-聚多巴胺组细胞外基质矿化水平强于纯钛组(P < 0.001,P < 0.000 1),纳米管-聚多巴胺组细胞外基质矿化水平强于纳米管组(P < 0.01)。该现象与碱性磷酸酶活性检测结果一致,证实表面功能化修饰可梯度调控大鼠骨髓间充质干细胞的成骨分化-矿化级联反应。 "
| [1] YUAN P, CHEN M, LU X, et al. Application of advanced surface modification techniques in titanium-based implants: latest strategies for enhanced antibacterial properties and osseointegration. J Mater Chem B. 2024;12(41):10516-10549. [2] HAN X, MA J, TIAN A, et al. Surface modification techniques of titanium and titanium alloys for biomedical orthopaedics applications: A review. Colloids Surf B Biointerfaces. 2023;227:113339. [3] HUO SC, YUE B. Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface. World J Stem Cells. 2020;12(7):545-561. [4] SOTOVA C, YANUSHEVICH O, KRIHELI N, et al. Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials. 2023;16(23):7383. [5] WANG S, ZHAO X, HSU Y, et al. Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta Biomater. 2023;169:19-44. [6] DONOHOE E, KAHATAB R, BARRAK F. A systematic review comparing the macrophage inflammatory response to hydrophobic and hydrophilic sandblasted large grit, acid‐etched titanium or titanium–zirconium surfaces during in vitro studies. Clin Exp Dent Res. 2023;9(3):437-448. [7] WEN X, LIU Y, XI F, et al. Micro-arc oxidation (MAO) and its potential for improving the performance of titanium implants in biomedical applications. Front Bioeng Biotechnol. 2023;11:1282590. [8] CHHABRA K, RAJASEKAR A. Comparison of Roughness, Wettability, and SEM Features between Sandblasted Acid-Etched and Oxidized Titanium Dental Implants. J Long Term Eff Med Implants. 2024;34(4):57-63. [9] ALEMAYEHU DB, TODOH M, HSIEH JH, et al. Improving Pure Titanium’s Biological and Mechanical Characteristics through ECAP and Micro-Arc Oxidation Processes. Micromachines. 2023;14(8):1541. [10] LI J, CUI X, HOOPER GJ, et al. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J Mech Behav Biomed Mater. 2020;105:103671. [11] JACOBS TW, DILLON JT, COHEN DJ, et al. Different Methods to Modify the Hydrophilicity of Titanium Implants with Biomimetic Surface Topography to Induce Variable Responses in Bone Marrow Stromal Cells. Biomimetics. 2024;9(4):227. [12] KIDO D, KOMATSU K, SUZUMURA T, et al. Influence of Surface Contaminants and Hydrocarbon Pellicle on the Results of Wettability Measurements of Titanium. Int J Mol Sci. 2023;24(19):14688. [13] REN B, WAN Y, LIU C, et al. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater Sci Eng C Mater Biol Appl. 2021; 118:111505. [14] WU N, GAO H, WANG X, et al. Surface Modification of Titanium Implants by Metal Ions and Nanoparticles for Biomedical Application. ACS Biomater Sci Eng. 2023;9(6):2970-2990. [15] ZHOU S, XIAO C, FAN L, et al. Injectable ultrasound-powered bone-adhesive nanocomposite hydrogel for electrically accelerated irregular bone defect healing. J Nanobiotechnology. 2024;22(1):54. [16] LI Y, XU C, LEI C. The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair. Pharmaceutics. 2023;15(3):1017. [17] YU YM, LU YP, ZHANG T, et al. Biomaterials science and surface engineering strategies for dental peri-implantitis management. Mil Med Res. 2024;11(1):29. [18] BANDYOPADHYAY A, SHIVARAM A, MITRA I, et al. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019;96:686-693. [19] LI J, MUTREJA I, TREDINNICK S, et al. Hydrodynamic control of titania nanotube formation on Ti-6Al-4V alloys enhances osteogenic differentiation of human mesenchymal stromal cells. Mater Sci Eng C Mater Biol Appl. 2020;109:110562. [20] MA QL, FANG L, JIANG N, et al. Bone mesenchymal stem cell secretion of sRANKL/OPG/M-CSF in response to macrophage-mediated inflammatory response influences osteogenesis on nanostructured Ti surfaces. Biomaterials. 2018;154:234-247. [21] GUO Q, CHEN J, WANG J, et al. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale. 2020;12(3):1307-1324. [22] JINSHENG L, QING D, JUNHAO C, et al. Micro/nano topological modification of TiO nanotubes activates Thy-1 signaling to control osteogenic differentiation of stem cells. SLAS Discov. 2024;29(3): 100139. [23] GUO X, BAI J, GE G, et al. Bioinspired peptide adhesion on Ti implants alleviates wear particle-induced inflammation and improves interfacial osteogenesis. J Colloid Interface Sci. 2022;605:410-424. [24] SAMYN P. A platform for functionalization of cellulose, chitin/chitosan, alginate with polydopamine: A review on fundamentals and technical applications. Int J Biol Macromol. 2021;178:71-93. [25] XIA S, LIU D, JIANG K, et al. Photothermal driven BMSCs osteogenesis and M2 macrophage polarization on polydopamine-coated TiC nanosheets/poly(vinylidene fluoride trifluoroethylene) nanocomposite coatings. Mater Today Bio. 2024;27:101156. [26] LEE H, DELLATORE SM, MILLER WM, et al. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science. 2007;318(5849): 426-430. [27] MA Q, JIANG N, LIANG S, et al. Functionalization of a clustered TiO nanotubular surface with platelet derived growth factor-BB covalent modification enhances osteogenic differentiation of bone marrow mesenchymal stem cells. Biomaterials. 2020;230:119650. [28] YANG X, WANG Q, YAN C, et al. A dual-functional strontium-decorated titanium implants that guides the immune response for osseointegration of osteoporotic rats. Colloids Surf B Biointerfaces. 2024;233:113643. [29] DING C, LU Y, XIANG M, et al. Internal electric field-assisted copper ions chelated polydopamine/titanium dioxide nano-thin film heterojunctions activate peroxymonosulfate under visible light to catalyze degradation of gatifloxacin: Theoretical calculations and biotoxicity analysis. J Colloid Interface Sci. 2023;646:275-289. [30] CHEN J, CHEN T, FANG Q, et al. Gd2 O3 /b‐TiO2 composite nanoprobes with ultra‐high photoconversion efficiency for MR image‐guided NIR‐II photothermal therapy. Exploration. 2022;2(6):20220014. [31] WEBSTER TJ. Reduced adhesion of macrophages on anodized titanium with select nanotube surface features. Int J Nanomedicine. Published online August 2011;6:1765-1771. [32] SEREDIN P, GOLOSHCHAPOV D, BUYLOV N, et al. A Study of the Peculiarities of the Formation of a Hybrid Interface Based on Polydopamine between Dental Tissues and Dental Composites, Using IR and Raman Microspectroscopy, at the Submicron Level. Int J Mol Sci. 2023;24(14):11636. [33] LU R, WANG C, WANG X, et al. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int J Nanomedicine. 2018;13:2037-2049. [34] SUN XD, LIU TT, WANG QQ, et al. Surface Modification and Functionalities for Titanium Dental Implants. ACS Biomater Sci Eng. 2023;9(8):4442-4461. [35] LI X, COMBS JD, SALAITA K, et al. Polarized focal adhesion kinase activity within a focal adhesion during cell migration. Nat Chem Biol. 2023;19(12):1458-1468. [36] XING B, LEI Z, WANG Z, et al. A disintegrin and metalloproteinase 22 activates integrin β 1 through its disintegrin domain to promote the progression of pituitary adenoma. Neuro-Oncol. 2024;26(1):137-152. [37] OLIVEIRA WF, ARRUDA IRS, SILVA GMM, et al. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mater Sci Eng C. 2017;81:597-606. [38] MA T, WANG C, GE X, et al. Applications of Polydopamine in Implant Surface Modification. Macromol Biosci. 2023;23(10):2300067. [39] HE R, SUI J, WANG G, et al. Polydopamine and hyaluronic acid immobilisation on vancomycin-loaded titanium nanotube for prophylaxis of implant infections. Colloids Surf B Biointerfaces. 2022; 216:112582. [40] OMIDIAN H, WILSON RL. Polydopamine Applications in Biomedicine and Environmental Science. Materials. 2024;17(16):3916. [41] ZHANG Q, PAN RL, WANG H, et al. Nanoporous Titanium Implant Surface Accelerates Osteogenesis via the Piezo1/Acetyl-CoA/β-Catenin Pathway. Nano Lett. 2024;24(27):8257-8267. [42] LIU C, LI Y, WANG J, et al. Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application. Molecules. 2018;23(7):1643. [43] WANG H, YUAN C, LIN K, et al. Modifying a 3D-Printed Ti6Al4V Implant with Polydopamine Coating to Improve BMSCs Growth, Osteogenic Differentiation, and In Situ Osseointegration In Vivo. Front Bioeng Biotechnol. 2021;9:761911. [44] ZHANG Y, DONG C, YANG S, et al. Enhanced silver loaded antibacterial titanium implant coating with novel hierarchical effect. J Biomater Appl. 2018;32(9):1289-1299. [45] XIANG Y, LIN D, ZHOU Q, et al. Elucidating the Mechanism of Large-Diameter Titanium Dioxide Nanotubes in Protecting Osteoblasts Under Oxidative Stress Environment: The Role of Fibronectin and Albumin Adsorption. Int J Nanomedicine. 2024;19:10639-10659. [46] JIA L, HAN F, WANG H, et al. Polydopamine-assisted surface modification for orthopaedic implants. J Orthop Transl. 2019;17:82-95. [47] VIMALRAJ S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene. 2020;754:144855. [48] BERNAR A, GEBETSBERGER JV, BAUER M, et al. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int J Mol Sci. 2022;24(1):723. [49] MORANDINI RODRIGUES L, LIMA ZUTIN EA, SARTORI EM, et al. Nanoscale hybrid implant surfaces and Osterix ‐mediated osseointegration. J Biomed Mater Res A. 2022;110(3):696-707. [50] FARJAMINEJAD S, FARJAMINEJAD R, GARCIA-GODOY F. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. J Funct Biomater. 2024;15(9):241. [51] KULKARNI M, MAZARE A, GONGADZE E, et al. Titanium nanostructures for biomedical applications. Nanotechnology. 2015;26(6):062002. [52] ZHAN J, LI L, YAO L, et al. Evaluation of sustained drug release performance and osteoinduction of magnetron-sputtered tantalum-coated titanium dioxide nanotubes. RSC Adv. 2024;14(6):3698-3711. |
| [1] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [2] | Jiang Xinghai, Song Yulin, Li Dejin, Shao Jianmin, Xu Junzhi, Liu Huakai, Wu Yingguo, Shen Yuehui, Feng Sicheng. Vascular endothelial growth factor 165 genes transfected into bone marrow mesenchymal stem cells to construct a vascularized amphiphilic peptide gel module [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1903-1911. |
| [3] | Yang Qiongqiong, Liu Wei. Comparison of performance and clinical effects of zirconia and titanium implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2063-2071. |
| [4] | Min Changqin, Huang Ying. Construction of pH/near-infrared laser stimuli-responsive drug delivery system and its application in treatment of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1940-1951. |
| [5] | Li Keyun, Yang Yuqi, Fei Yingying, Huang Shuai. Physicochemical properties and in vitro biological effects of resveratrol-eluting stents [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5243-5256. |
| [6] | Li Shu, Zhao Zhengyi, Zeng Qin, Zhu Xiangdong. Nanohydroxyapatite induces immunogenic cell death in tumors [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5143-5151. |
| [7] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [8] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [9] | Wu Tianyi, Miao Yiming, Wan Kaichen, Teng Yun, Zou Jun. Protective effect of mesoporous ZLN005@polydopamine nanoparticles on chondrocytes in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3576-3585. |
| [10] | Zhao Shuai, Li Dongyao, Wei Suiyan, Cao Yijing, Xu Yan, Xu Guoqiang. Biocompatibility of poly(vinylidene fluoride) piezoelectric bionic periosteum prepared by electrospinning [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 730-737. |
| [11] | He Rui, Li Chongyi, Wang Ruiyao, Zeng Dan, Fan Daidi. Application of MXene-based hydrogels in wound repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3486-3493. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||