Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5243-5256.doi: 10.12307/2026.158
Previous Articles Next Articles
Physicochemical properties and in vitro biological effects of resveratrol-eluting stents
Li Keyun, Yang Yuqi, Fei Yingying, Huang Shuai
- School of Life Science and Engineering, Southwest Jiaotong University, Chengdu 611756, Sichuan Province, China
-
Accepted:2025-05-06Online:2026-07-18Published:2025-11-27 -
Contact:Huang Shuai, PhD, Associate professor, School of Life Science and Engineering, Southwest Jiaotong University, Chengdu 611756, Sichuan Province, China -
About author:Li Keyun, MS, School of Life Science and Engineering, Southwest Jiaotong University, Chengdu 611756, Sichuan Province, China
CLC Number:
Cite this article
Li Keyun, Yang Yuqi, Fei Yingying, Huang Shuai. Physicochemical properties and in vitro biological effects of resveratrol-eluting stents[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5243-5256.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
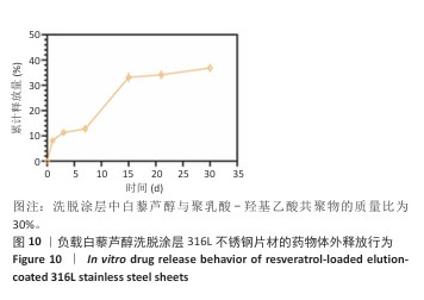
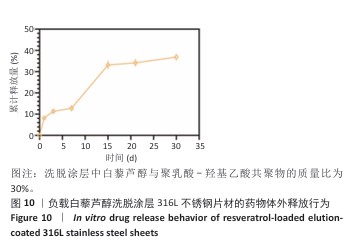
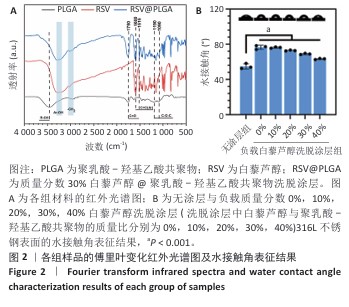
2.1 白藜芦醇洗脱涂层的材料学表征结果 通过傅里叶变换红外光谱对涂层的化学结构进行分析,结果如图2A所示。聚乳酸-羟基乙酸共聚物的红外谱图显示在3 464 cm-1处出现了明显的氧氢峰,在1 750 cm-1处观察到聚乳酸-羟基乙酸共聚物酯键中羰基的伸缩振动特征峰,同时在1 190 cm-1和1 090 cm-1处出现了C-O-C的伸缩振动。白藜芦醇的红外光谱图显示在3 200-3 400 cm-1处出现了宽而强的酚羟基峰,归因于白藜芦醇中多个酚羟基基团的贡献,在1 600 cm-1和1 515 cm-1处出现了明显的苯环骨架C=C的伸缩振动。在白藜芦醇@聚乳酸-羟基乙酸共聚物中,分别出现了聚乳酸-羟基乙酸共聚物和白藜芦醇分子的特征吸收峰,并且白藜芦醇中宽而强的O-H吸收带减弱并略微位移,表明白藜芦醇与聚乳酸-羟基乙酸共聚物通过酚羟基和酯基之间的相互作用实现了结合,证实了洗脱涂层的成功构建,同时也表明聚乳酸-羟基乙酸共聚物有效实现了对白藜芦醇分子的物理包载,并保持了白藜芦醇结构的完整性。 对涂层的润湿性进行了水接触角测试,结果展示如图2B。与316L不锈钢片材相比,负载0%白藜芦醇洗脱涂层的316L不锈钢片材水接触角显著增大,达到了(76.53±2.59)°,这种变化归因于聚乳酸-羟基乙酸共聚物中富含疏水性基团,使得材料表面对水的润湿性降低,接触角增大。而对于负载白藜芦醇洗脱涂层的316L不锈钢片,随着白藜芦醇装载量的增加,材料表面的水接触角逐步减小,这种现象可能是由于白藜芦醇分子中含有多个羟基(-OH),这些羟基能够与水分子形成氢键,从而增强了材料表面的亲水性,进一步证明了白藜芦醇@聚乳酸-羟基乙酸共聚物涂层成功制备并具有明确的特性。"
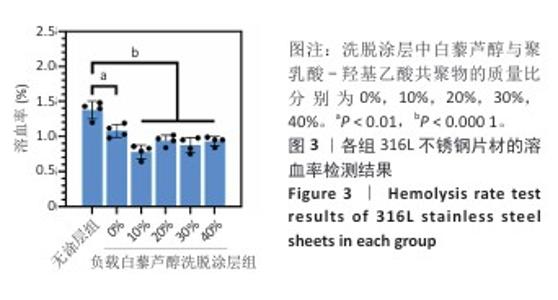
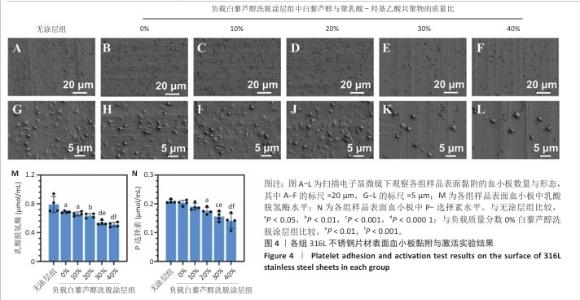
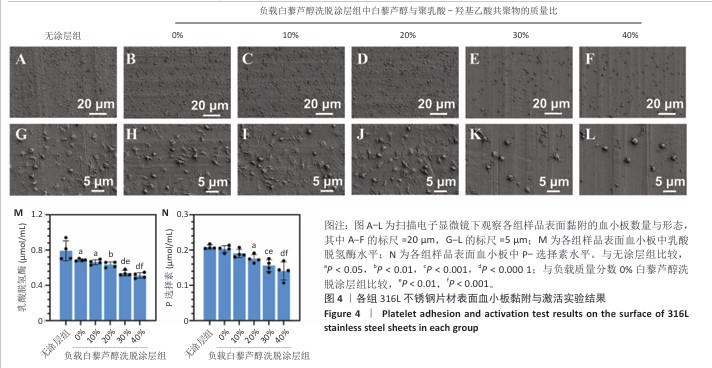
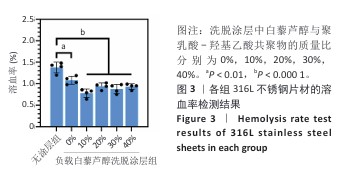
2.2 白藜芦醇洗脱涂层的体外血液相容性评价结果 溶血率直接反映材料对红细胞的破坏性,是判断材料是否适合与血液长期接触的重要指标。各组样品的溶血实验结果如图3所示。316L不锈钢片材与负载质量分数0%,10%,20%,30%,40%白藜芦醇洗脱涂层316L不锈钢片材的溶血率低于国际标准(溶血率< 5%),表明白藜芦醇洗脱涂层材料具有良好的血液相容性。其中,白藜芦醇与聚乳酸-羟基乙酸共聚物基质的结合可能进一步降低了材料表面对红细胞的破坏性,使红细胞在接触涂层表面时保持完整性,减少了膜损伤和溶血的发生。 支架植入体内过程中直接与血液接触,这种界面相互作用对血栓形成和支架功能的长期效果具有决定性影响。血小板黏附和活化程度是评价血管植入物血液相容性的重要指标[43],因此,此次研究通过体外血小板黏附与激活实验评估涂层材料的性能。有研究表明,白藜芦醇能够显著抑制血小板黏附和激活[44-45]。各组样品表面的血小板黏附与激活实验结果如图4显示。扫描电子显微镜观察结果显示,相较于无涂层316L不锈钢片材,负载涂层的316L不锈钢片材表面血小板黏附数量减少,同时保持了血小板的完整形态,未观察到明显的伪足延伸或形态改变,并且随着涂层中白藜芦醇质量分数的增加,血小板黏附数量减少。相较于无涂层316L不锈钢片材组,负载质量分数0%,10%,20%,30%,40%白藜芦醇洗脱涂层316L不锈钢片材组乳酸脱氢酶水平降低,负载质量分数20%,30%,40%白藜芦醇洗脱涂层316L不锈钢片材组P-选择素水平降低,负载质量分数30%,40%白藜芦醇洗脱涂层316L不锈钢片材组乳酸脱氢酶与P-选择素水平均低于负载质量分数0%白藜芦醇洗脱涂层316L不锈钢片材组,差异均有显著性意义,表明白藜芦醇洗脱涂层有效抑制了血小板的黏附与活化。 "
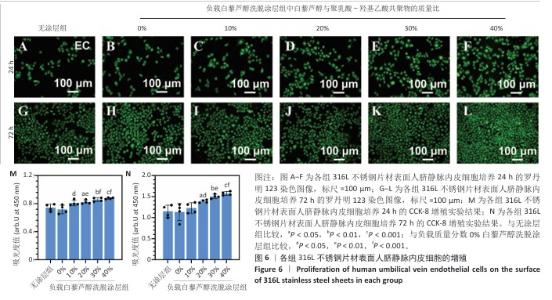
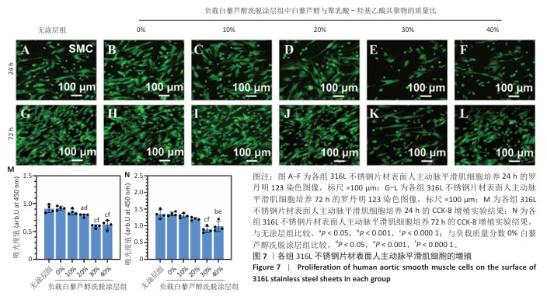
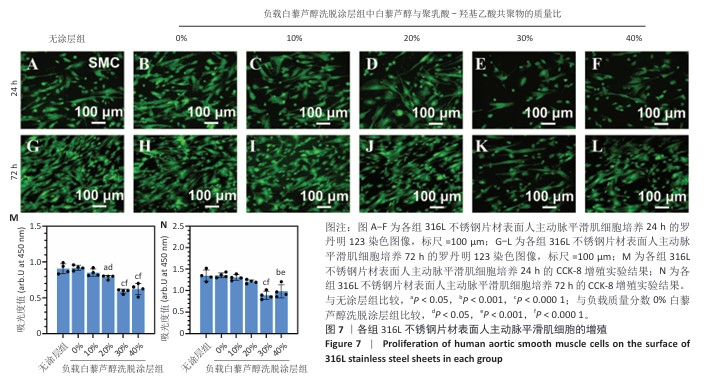
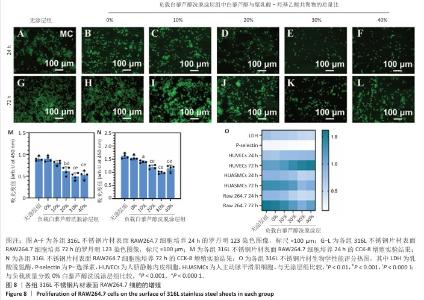
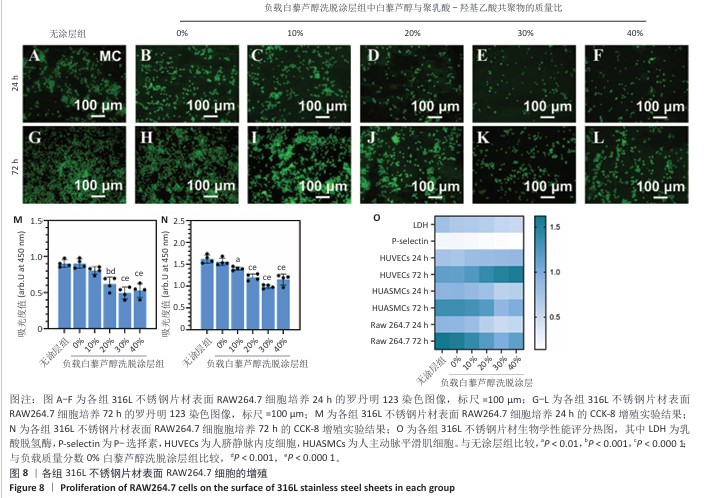
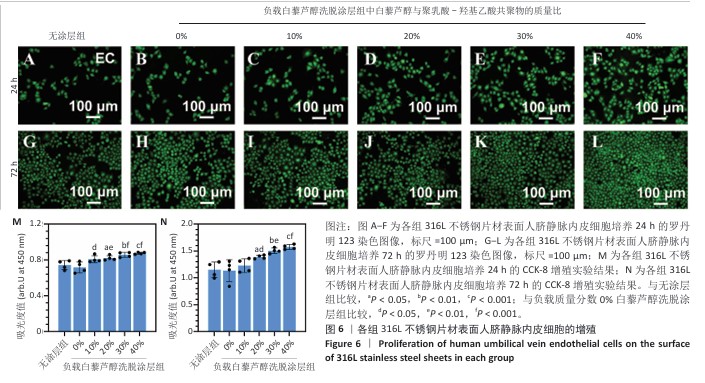
2.4 白藜芦醇洗脱涂层的体外细胞相容性评估结果 支架植入后,内皮细胞损伤会导致内膜修复延迟,增加血栓形成的风险。研究表明,白藜芦醇能够激活内皮一氧化氮合酶、提高一氧化氮水平,促进血管舒张和内皮修复[37];此外,白藜芦醇可抑制氧化应激损伤[31],从而保护内皮细胞功能,增强增殖能力。图6展示了人脐静脉内皮细胞在各组样品表面的增殖检测结果。罗丹明123染色结果显示,相同培养时间下,随着涂层中白藜芦醇质量分数的增加,人脐静脉内皮细胞数量增加。CCK-8检测结果显示,相同培养时间下,负载质量分数20%,30%,40%白藜芦醇洗脱涂层组人脐静脉内皮细胞增殖多于无涂层组,差异有显著性意义;培养24 h,负载质量分数10%,20%,30%,40%白藜芦醇洗脱涂层组人脐静脉内皮细胞增殖多于负载质量分数0%白藜芦醇洗脱涂层组,差异有显著性意义;培养72 h,负载质量分数20%,30%,40%白藜芦醇洗脱涂层组人脐静脉内皮细胞增殖多于负载质量分数0%白藜芦醇洗脱涂层组,差异有显著性意义。表明白藜芦醇能够显著促进内皮细胞的增殖,进而促进血管再生或修复。 支架植入后,平滑肌细胞的异常增殖是支架内再狭窄的主要原因[46-47]。研究表明,白藜芦醇通过阻滞细胞周期(G1或S期)抑制与增殖相关的信号通路(磷脂酰肌醇3-激酶/蛋白激酶B和丝裂原活化蛋白激酶信号通路),降低促增殖因子的表达(血小板源性生长因子)[48-49]。图7展示了人主动脉平滑肌细胞在各组样品表面的增殖检测结果。罗丹明123染色结果显示,相同培养时间下,随着涂层中白藜芦醇质量分数的增加,人主动脉平滑肌细胞数量呈减少趋势。CCK-8检测结果显示,培养24 h,负载质量分数20%,30%,40%白藜芦醇洗脱涂层组人主动脉平滑肌细胞增殖少于无涂层组、负载质量分数0%白藜芦醇洗脱涂层组,差异有显著性意义;培养72 h,负载质量分数30%,40%白藜芦醇洗脱涂层组人主动脉平滑肌细胞增殖少于无涂层组、负载质量分数0%白藜芦醇洗脱涂层组差异有显著性意义。以上结果表明,白藜芦醇可抑制平滑肌细胞过度增殖,可能有助于防止支架植入后内膜增生和再狭窄的发生。 从上述荧光图和CCK-8实验结果可以看出,316L不锈钢片材与负载聚乳酸-羟基乙酸共聚物涂层的316L不锈钢片材对内皮细胞和平滑肌细胞增殖的影响较为平稳,未产生显著的促进或抑制作用。负载白藜芦醇洗脱涂层的316L不锈钢片材对内皮细胞增殖有促进作用,对平滑肌细胞增殖有抑制作用,推测这种效果主要来源于聚乳酸-羟基乙酸共聚物涂层中白藜芦醇的缓释,而非聚乳酸-羟基乙酸共聚物本身。结果表明,白藜芦醇作为洗脱涂层中的活性成分,发挥了促进内皮细胞增殖和抑制平滑肌细胞增殖的作用,从而提高了涂层的生物相容性和治疗效果。 巨噬细胞在支架植入后的炎症反应中起关键作用,巨噬细胞过度增殖会导致慢性炎症,影响支架的长期稳定性。研究表明,白藜芦醇通过调控巨噬细胞增殖可显著降低炎症水平,改善支架植入后的组织修复环境[50]。图8展示了RAW264.7细胞在各组样品表面的增殖检测结果。罗丹明123染色结果显示,相同培养时间下,随着涂层中白藜芦醇质量分数的增加,RAW264.7细胞数量呈减少趋势。CCK-8检测结果显示,培养24 h,负载质量分数20%,30%,40%白藜芦醇洗脱涂层组RAW264.7细胞增殖少于无涂层组、负载质量分数0%白藜芦醇洗脱涂层组,差异有显著性意义;培养72 h,负载质量分数10%,20%,30%,40%白藜芦醇洗脱涂层组RAW264.7细胞增殖少于无涂层组,负载质量分数20%,30%,40%白藜芦醇洗脱涂层组RAW264.7细胞增殖少于负载质量分数0%白藜芦醇洗脱涂层组,差异均有显著性意义。 对血小板黏附与激活、人脐静脉内皮细胞、人主动脉平滑肌细胞和巨噬细胞增殖实验数据进行归一化处理,并绘制热图,结果如图8显示,发现质量分数30%白藜芦醇洗脱涂层表现出最佳的综合性能,因此,选择质量分数30%白藜芦醇洗脱涂层进行后续实验。 "
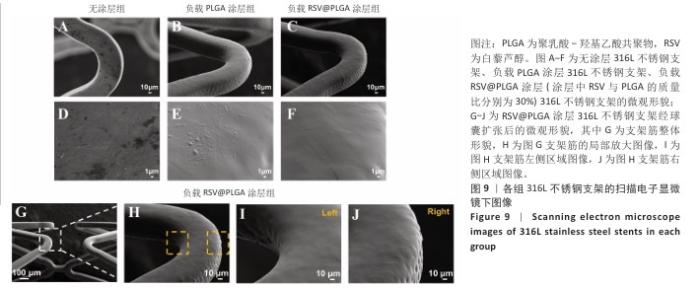
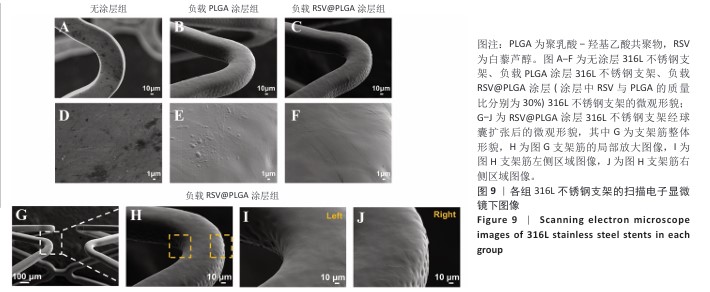
2.5 白藜芦醇洗脱涂层的机械性能测试结果 血管支架在体内应用时需要承受体内不同的物理负荷,包括血流的压力、组织的拉伸等,支架需要具有适应这些应力变化的能力,避免因力学性能不足而发生形变或失效。研究表明药物洗脱支架能够达到体内负荷要求[51]。当支架发生形变时,若涂层缺乏足够的延展性,可能会出现开裂或剥离现象,进而导致药物释放不均或失效,甚至损害支架的生物相容性和功能完整性。各组样品的扫描电子显微镜下图像,见图9A-F。316L不锈钢支架表面呈现典型的金属加工形貌,负载0%白藜芦醇洗脱涂层(单纯的聚乳酸-羟基乙酸共聚物涂层)316L不锈钢支架表面形成连续、无裂纹的聚合物薄膜,完全覆盖金属基底,负载质量分数30%白藜芦醇洗脱涂层316L不锈钢支架表面形貌与负载0%白藜芦醇洗脱涂层316L不锈钢支架基本一致,表明药物负载未引起相分离或孔洞缺陷,并且表面均匀一致,未见明显破损或厚度不均的情况。经球囊扩张316L不锈钢支架后,白藜芦醇洗脱涂层未出现涂层剥落、裂缝扩展等问题,能够适应血管支架在应用中的变形要求,见图9G-J。 "
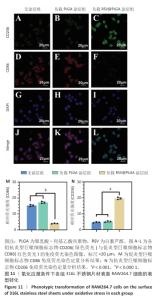
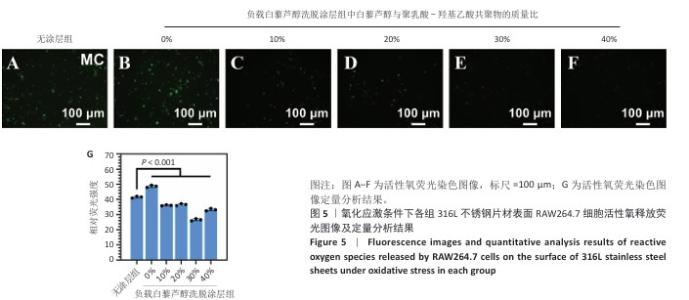
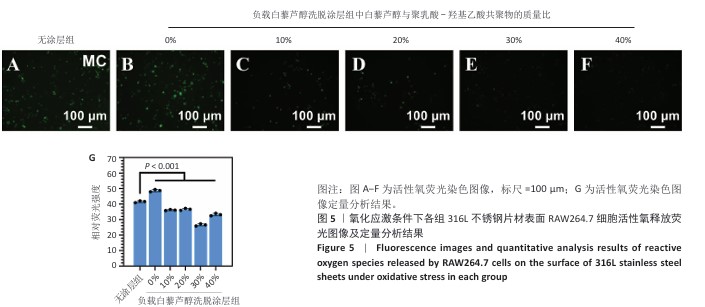
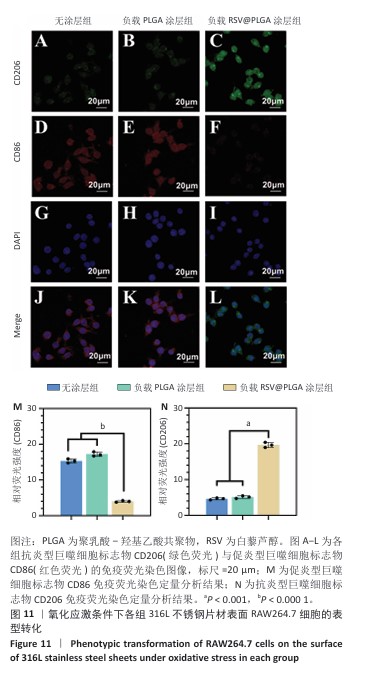
2.7 白藜芦醇洗脱涂层对巨噬细胞表型的影响实验结果 动脉粥样硬化是一种慢性关键特征的复杂病理过程,而巨噬细胞通过其功能可塑性发挥核心调控作用[52-54],这类免疫细胞表现出的表型可塑性转变(促炎型巨噬细胞与抗炎型巨噬细胞)深刻影响着斑块的形成机制、进展过程及结构稳定性[55-56],其中促炎型巨噬细胞通过分泌促炎递质(肿瘤坏死因子α、白细胞介素6)和活性氧加剧血管内皮损伤并促进脂质异常沉积,从而驱动易损斑块的形成;抗炎型巨噬细胞能够依赖抗炎因子(白细胞介素10)和促进组织修复的生长因子(转化生长因子β),在促进斑块区域组织修复、调控脂质代谢平衡以及增强斑块结构稳定性等方面产生保护性效应[57-58]。 氧化应激条件下各组样品表面RAW264.7细胞的表型转化结果,见图11所示。免疫荧光染色结果显示,负载质量分数30%白藜芦醇洗脱涂层组促炎型巨噬细胞标志物CD86表达低于无涂层组、负载质量分数0%白藜芦醇洗脱涂层组(P < 0.000 1),抗炎型巨噬细胞标志物CD206表达高于无涂层组、负载质量分数0%白藜芦醇洗脱涂层组(P < 0.001),表明白藜芦醇洗脱涂层能够促进巨噬细胞向抗炎表型转化。因此,白藜芦醇洗脱涂层能够有效调整巨噬细胞的促炎型巨噬细胞/抗炎型巨噬细胞比例,从促进炎症状态向抗炎、修复状态转化。 "
| [1] BJÖRKEGREN JLM, LUSIS AJ. Atherosclerosis: Recent developments. Cell. 2022;185(10):1630-1645. [2] LIBBY P. The changing landscape of atherosclerosis. Nature. 2021; 592(7855):524-533. [3] TSAO CW, ADAY AW, ALMARZOOQ ZI, et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circul Res. 2023;147(8):e93-e621. [4] JIA S, LIU Y, YUAN J. Evidence in Guidelines for Treatment of Coronary Artery Disease. Adv Exp Med Biol. 2020;1177:37-73. [5] MONTRIEF T, KOYFMAN A, LONG B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am J Emerg Med. 2018;36(12):2289-2297. [6] HOOLE SP, BAMBROUGH PJH. Recent advances in percutaneous coronary intervention. Heart. 2020;106(18):1380-1386. [7] DOENST T, HAVERICH A, SERRUYS P, et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73(8):964-976. [8] HONG SJ, HONG MK. Drug-eluting stents for the treatment of coronary artery disease: A review of recent advances. Expert Opin Drug Deliv. 2022;19(3):269-280. [9] LEE DH, DE LA TORRE HERNANDEZ JM. The Newest Generation of Drug-eluting Stents and Beyond. Eur Cardiol. 2018;13(1):54-59. [10] TORII S, JINNOUCHI H, SAKAMOTO A, et al. Drug-eluting coronary stents: insights from preclinical and pathology studies. Nat Rev Cardiol. 2020;17(1):37-51. [11] BAAN J JR, CLAESSEN BE, DIJK KB, et al. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the DARE trial. JACC Cardiovasc Interv. 2018;11(3):275-283. [12] WANG R, LU J, YIN J, et al. A TEMPOL and rapamycin loaded nanofiber-covered stent favors endothelialization and mitigates neointimal hyperplasia and local inflammation. Bioact Mater. 2023;19(1):666-677. [13] UDRIȘTE AS, BURDUȘEL AC, NICULESCU AG, et al. Coatings for cardiovascular stents—an up-to-date review. Int J Mol Sci. 2024; 25(2):1078. [14] DU R, WANG Y, HUANG Y, et al. Design and testing of hydrophobic core/hydrophilic shell nano/micro particles for drug-eluting stent coating. NPG Asia Mater. 2018;10(7):642-658. [15] LATTUCA B, ODORICO X, OCCEAN B, et al. Long-term efficacy and safety of newer generation ultrathin strut drug-eluting stents: a systematic review and meta-analysis. Eur Heart J. 2020; 41(Supplement_2):ehaa946.2543. [16] HAUDE M, INCE H, ABIZAID A, et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387(10013):31-39. [17] MOUSAVIZADEH SM, YU M, GILCHRIST MD, et al. Preparation of a polycaprolactone coating on WE43 for biodegradable stent applications using dual solvents, ultrasonic atomization spray, and anodization. Prog Org Coat. 2024;193(8):108528. [18] HERMAWAN H, DUBE D, MANTOVANI D. Developments in metallic biodegradable stents. Acta Biomater. 2010;6(5):1693-1697. [19] HU X, ZHAO W, ZHANG Z, et al. Novel 3D printed shape-memory PLLA-TMC/GA-TMC scaffolds for bone tissue engineering with the improved mechanical properties and degradability. Chin Chem Lett. 2023;34(1):107451. [20] WEN Y, LI Y, YANG R, et al. Biofunctional coatings and drug-coated stents for restenosis therapy. Mater Today Bio. 2024;29(6):101259. [21] BARTOSCH M, SCHUBERT S, BERGER FJB. Magnesium stents–fundamentals, biological implications and applications beyond coronary arteries. Bionanomaterials. 2015;16(1):3-17. [22] TOONG DWY, NG JCK, HUANG Y, et al. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia. 2020;12(4):100727. [23] ALEXY RD, LEVI DS. Materials and manufacturing technologies available for production of a pediatric bioabsorbable stent. Biomed Res Int. 2013;2013(1):137985. [24] O’BRIEN B, ZAFAR H, IBRAHIM A, et al. Coronary stent materials and coatings: a technology and performance update. Ann Biomed Eng. 2016;44(2):523-535. [25] KIRILLOVA A, YEAZEL TR, ASHEGHALI D, et al. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem Rev. 2021; 121(18):11238-11304. [26] BLASI P. Poly (lactic acid)/poly (lactic-co-glycolic acid)-based microparticles: An overview. J Pharm Investig. 2019;49(4):337-346. [27] LEE PC, ZAN BS, CHEN LT, et al. Multifunctional PLGA-based nanoparticles as a controlled release drug delivery system for antioxidant and anticoagulant therapy. Int J Nanomed. 2019;14:1533-1549. [28] BUTT MA. Thin-film coating methods: a successful marriage of high-quality and cost-effectiveness—a brief exploration. Coatings. 2022;12(8):1115. [29] LI J, HU X, CHEN Y, et al. Review of recent progress in vascular stents: From conventional to functional vascular stents. Chin Chem Lett. 2024;35(1):110492. [30] WU X, WYMAN I, ZHANG G, et al. Preparation of superamphiphobic polymer-based coatings via spray-and dip-coating strategies. Prog Org Coat. 2016;90(1):463-471. [31] MUTHIAH P, BHUSHAN B, YUN K, et al. Dual-layered-coated mechanically-durable superomniphobic surfaces with anti-smudge properties. J Colloid Interface Sci. 2013;409(21):227-236. [32] BOSE S, KELLER SS, ALSTROM TS, et al. Process optimization of ultrasonic spray coating of polymer films. Langmuir. 2013;29(23): 6911-6919. [33] GOGATE P, KHAIRE R. Use of ultrasonic atomization for encapsulation and other processes in food and pharmaceutical manufacturing. Power ultrasonics, 2023:773-794. [34] NAIDU H, KAHRAMAN O, FENG H. Novel applications of ultrasonic atomization in the manufacturing of fine chemicals, pharmaceuticals, and medical devices. Ultrason Sonochem. 2022;86(5):105984. [35] PHAM NP, BOELLAARD E, BURGHARTZ JN, et al. Photoresist coating methods for the integration of novel 3-D RF microstructures.J Microelectromech Syst. 2004;13(3):491-499. [36] GAL R, DERES L, TOTH K, et al. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int J Mol Sci. 2021;22(18):10152. [37] PARSAMANESH N, ASGHARI A, SARDARI S, et al. Resveratrol and endothelial function: A literature review. Pharmacol Res. 2021;170(8): 105725. [38] LI H, XIA N, HASSELWANDER S, et al. Resveratrol and Vascular Function. Int J Mol Sci. 2019;20(9):2155. [39] BREUSS JM, ATANASOV AG, UHRIN P. Resveratrol and Its Effects on the Vascular System. Int J Mol Sci. 2019;20(7):1523. [40] PENG Y, ZHENG X, ZHANG S, et al. Advances in the activity of resveratrol and its derivatives in cardiovascular diseases. Arch Pharm (Weinheim). 2025;358(2):e2400865. [41] MALAGUARNERA L. Influence of Resveratrol on the Immune Response. Nutrients. 2019;11(5):946. [42] PAGE MJ, KELL DB, PRETORIUS E.The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress (Thousand Oaks). 2022;6:24705470221076390. [43] WEBER M, STEINLE H, GOLOMBEK S, et al. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front Bioeng Biotechnol. 2018;6(16):99. [44] MICHNO A, GRUZEWSKA K, RONOWSKA A, et al. Resveratrol Inhibits Metabolism and Affects Blood Platelet Function in Type 2 Diabetes. Nutrients. 2022;14(8):1633. [45] MARUMO M, EKAWA K, WAKABAYASHI I. Resveratrol inhibits Ca(2+) signals and aggregation of platelets. Environ Health Prev Med. 2020; 25(1):70. [46] NANKIVELL V, PRIMER K, VIDANAPATHIRANA A, et al. Vascular biology of smooth muscle cells and restenosis. Mechanisms of vascular disease: A textbook for vascular specialists, 2020:117-139. [47] EL-MOKADEM M, EL-RAMLY M, HASSAN A, et al. Comparison between catheter-based delivery of paclitaxel after bare-metal stenting and drug-eluting stents in coronary artery disease patients at high risk for in-stent restenosis. Cardiovasc Revascula. 2017;18(8):596-600. [48] WANG Y, LEI L, SU Q, et al. Resveratrol Inhibits Insulin‐Induced Vascular Smooth Muscle Cell Proliferation and Migration by Activating SIRT1. Evid Based Complement Alternat Med. 2022;2022(1):8537881. [49] ASADPOUR S, YEGANEH H, KHADEMI F, et al. Resveratrol-loaded polyurethane nanofibrous scaffold: viability of endothelial and smooth muscle cells. Biomed Mater. 2019;15(1): 015001. [50] LI Y, FENG L, LI G, et al. Resveratrol prevents ISO-induced myocardial remodeling associated with regulating polarization of macrophages through VEGF-B/AMPK/NF-kB pathway. Int Immunopharmacol. 2020;84(7):106508. [51] CHICHAREON P, KATAGIRI Y, ASANO T, et al. Mechanical properties and performances of contemporary drug-eluting stent: focus on the metallic backbone. Expert Rev Med Devic. 2019;16(3):211-228. [52] BARRETT TJ. Macrophages in Atherosclerosis Regression. Arterioscler Thromb Vasc Biol. 2020;40(1):20-33. [53] BLAGOV AV, MARKIN AM, BOGATYREVA AI, et al. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells. 2023; 12(4):522. [54] HOU P, FANG J, LIU Z, et al. Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 2023;14(10):691. [55] MURRAY PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79(1): 541-566. [56] YUNNA C, MENGRU H, LEI W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;15(877):173090. [57] JINNOUCHI H, GUO L, SAKAMOTO A, et al. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell Mol Life Sci. 2020; 77(10):1919-1932. [58] HUANG X, LI Y, FU M, et al. Polarizing Macrophages In Vitro. Methods Mol Biol. 2018;1784:119-126. [59] KOŹLIK M, HARPULA J, CHUCHRA PJ, et al. Drug-eluting stents: Technical and clinical progress. Biomimetics. 2023;8(1):72. [60] SCAFA UDRIȘTE A, NICULESCU AG, GRUMEZESCU AM, et al. Cardiovascular stents: a review of past, current, and emerging devices. Materials. 2021;14(10):2498. [61] CONDELLO F, SPACCAROTELLA C, SORRENTINO S, et al. Stent Thrombosis and Restenosis with Contemporary Drug-Eluting Stents: Predictors and Current Evidence. J Clin Med. 2023;12(3):1238. [62] HASSAN S, ALI MN, GHAFOOR B. Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach. J Cardiothorac Surg. 2022;17(1):65. [63] NOGIC J, MCCORMICK LM, FRANCIS R, et al. Novel bioabsorbable polymer and polymer-free metallic drug-eluting stents. J Cardiol. 2018;71(5):435-443. [64] WANG C, LV J, YANG M, et al. Recent advances in surface functionalization of cardiovascular stents. Bioact Mater. 2025;44(2): 389-410. [65] LIU L, LAN X, CHEN X, et al. Multi-functional plant flavonoids regulate pathological microenvironments for vascular stent surface engineering. Acta Biomater. 2023;157(3):655-669. [66] LIU Y, SHI Y, ZHANG M, et al. Natural polyphenols for drug delivery and tissue engineering construction: A review. Eur J Med Chem. 2024; 266(4):116141. [67] 张晓琨,张晓晴,伍芳.超声雾化喷涂工艺制备醋酸纤维素多孔膜[J].电子元件与材料,2018,37(10):67-72. |
| [1] | Yang Qiongqiong, Liu Wei. Comparison of performance and clinical effects of zirconia and titanium implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2063-2071. |
| [2] | Wang Zhenze, Liu Fende, Zhang Rui, Li Wujun. Mesenchymal stem cells in treatment of arteriosclerosis obliterans of lower extremities: systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1869-1876. |
| [3] | Yao Yinxuan, Wen Suru, Chen Chaosheng, Wen Xin, Feng Keying, Kuang Zaoyuan, Zhang Wen. Puerarin-loaded injectable double-network hydrogel for promoting skin wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5201-5213. |
| [4] | Li Shu, Zhao Zhengyi, Zeng Qin, Zhu Xiangdong. Nanohydroxyapatite induces immunogenic cell death in tumors [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5143-5151. |
| [5] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [6] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [7] | Zhao Zheng. Diselenium contained polyurethane coatings with dual functions of anticoagulation and drug release [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 414-423. |
| [8] | Yang Lei, Liu Xinfang, Luo Sidong, Zhang Hongan, Wang Yeyang, Chen Weijian. Different preparation methods of silk fibroin and its application in the construction of small-diameter tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3694-3701. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||