Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (8): 2063-2071.doi: 10.12307/2026.561
Previous Articles Next Articles
Comparison of performance and clinical effects of zirconia and titanium implants
Yang Qiongqiong, Liu Wei
- Department of Stomatology, Chengdu Fifth People’s Hospital, Chengdu 610000, Sichuan Province, China
-
Received:2024-11-29Accepted:2025-01-25Online:2026-03-18Published:2025-07-24 -
Contact:Liu Wei, Chief physician, Department of Stomatology, Chengdu Fifth People’s Hospital, Chengdu 610000, Sichuan Province, China -
About author:Yang Qiongqiong, Physician, Department of Stomatology, Chengdu Fifth People’s Hospital, Chengdu 610000, Sichuan Province, China
CLC Number:
Cite this article
Yang Qiongqiong, Liu Wei. Comparison of performance and clinical effects of zirconia and titanium implants[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2063-2071.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
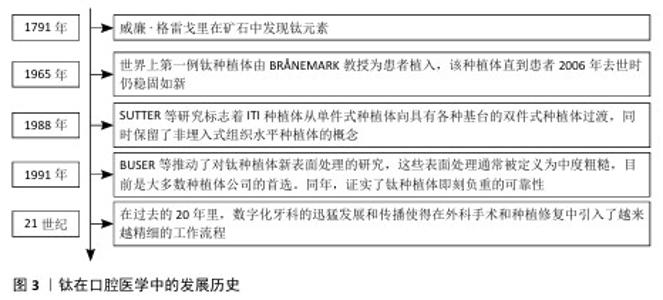
2.1 钛和氧化锆概述 2.1.1 钛 钛在口腔医学中的发展历史见图3。钛是第九大丰富金属,由威廉·格雷戈里于1791年发现。纯钛呈银白色,具有独特的物理化学特性,例如密度低(4 506 g/cm3)和强度高(590 MPa)[10]。1965年,世界上第一例钛种植体由BR?NEMARK教授为患者植入,该种植体直到患者2006年去世时仍稳固如新[11]。随后,SUTTER等在1988年的研究标志着 ITI 种植体从单件式种植体向具有各种基台的双件式种植体的过渡,同时保留了非埋入式组织水平种植体的概念[12]。20世纪90年代,BUSER等的研究结果推动了钛种植体新型表面处理的研究[13], 这些新型表面处理通常被定义为中度粗糙,目前是大多数种植体公司的首选。在同一年,种植牙功能负重前的等待时间得到了修订。长期随访和临床回顾研究证实了钛种植体即刻负重的可靠性[14]。进入21世纪后,数字化牙科的迅猛发展和传播使得在外科手术和种植修复中引入了越来越精细的工作流程[15]。钛可以快速与氧气发生反应在金属表面形成氧化层,因此具有抗腐蚀性。这种金属的研究涉及非常广泛的主题,例如在体育、颜料、珠宝、船舶设备、航空航天和医疗行业中的应用[16]。在牙科行业,钛及其合金以无毒著称,甚至比铬钴和不锈钢更具生物相容性[17]。钛金属具有不同的特性,在与牙科植入物相关的研究中氧化钛是报道最多的[18]。氧化钛是由钛金属与空气反应形成的氧化物,具有很强的抗腐蚀能力[19], 这层氧化层使得钛具有生物相容性[20]。目前,有6种不同类型的钛可用作植入生物材料,其中4种是商业纯钛等级(Ⅰ级、Ⅱ级、Ⅲ级和Ⅳ级),即98%-99.6%的纯钛,还有两种是钛合金(Ti-6Al-4V和Ti-6Al-4V-超低间隙合金),这些材料在耐腐蚀性、强度和延展性方面有所不同[21]。添加到钛中的合金元素主要分为阿尔法(α)稳定剂(例如铝、氧、氮和碳)与贝塔(β)稳定剂(例如钒、铁、镍和钴),因此,牙科钛合金有3种结构形式:α、β或两者的组合(α-β)[22]。其中,Ⅳ级钛和α-β组合合金(Ti-6Al-4V)在牙科应用中最广泛。钛种植体的表面非常重要,因为它们会影响与骨的相互作用。用作牙种植体主要材料(商业纯钛和Ti-6Al-4V)的表面由氧化钛组成,这种氧化物具有很高的耐腐蚀性,临床种植成功率高达99%[23]。 "
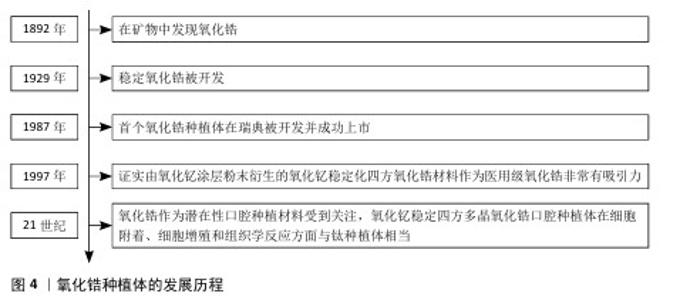
2.1.2 氧化锆 氧化锆种植体的发展历程见图4。氧化锆作为一种矿物于1892年被发现。稳定氧化锆于 1929年开发[24],具有良好的机械、光学和生物性能[25]。氧化锆的抗断裂性和弯曲强度明显高于氧化铝,使氧化锆植入物能够承受咬合力[26],成为制造陶瓷牙种植体的首选材料。因此,1987年,首个氧化锆种植体在瑞典被开发并成功上市。多态氧化锆结构以3种不同的晶体形式存在:单斜、四方和立方。在室温下,氧化锆获得单斜结构,在1 170 ℃时变为四方,在2 370 ℃ 时变为立方。得益于马氏体增韧机制[27],四方结构具有优异的断裂韧性和抗弯强度。四方相和立方相在室温下不稳定,冷却时会崩解,尽管如此,可通过添加CaO、MgO和Y2O3将氧化锆稳定在其立方相,从而得到一种称为部分稳定氧化锆的材料。1997年,研究者通过在室温下添加Y2O3获得四方氧化锆多晶体。氧化钇稳定化四方氧化锆的孔隙率低、密度高、弯曲强度和压缩强度高、断裂韧性高、抗疲劳性强,除了良好的机械性能外,它们还能促进骨整合过程中成骨细胞的增殖[28-29],适合医疗应用。人们通常认为氧化锆植入物不如钛植入物,因为人们担心氧化锆植入物会因断裂而取出[30]。由氧化铝增韧氧化锆制成的植入物已被证明比由氧化钇稳定化四方氧化锆制成的植入物更耐断裂[31]。与钛相比,氧化锆具有较低的表面能和表面润湿性,可能与更少的生物膜形成有关,从而进一步降低种植体周围炎的风险[32-33]。研究证明,钛种植体上早期形成(3 d)的生物膜量多于氧化锆种植体,两种材料种植体上14 d形成的生物膜量相当[32]。粗糙或疏水的表面通常会增加细菌黏附,这表明牙种植体的不同表面处理会影响生物膜的形成[34]。进入21世纪以来,氧化锆作为潜在性口腔种植材料受到关注,氧化钇稳定四方多晶氧化锆口腔种植体在细胞附着、细胞增殖和组织学反应方面与钛种植体相当[35]。"

2.2 钛与氧化锆的力学性能比较 氧化锆与钛种植体在力学性能方面的表现是评估其临床应用效果的重要指标。这两种材料在强度、弹性模量、硬度等关键参数上展现出了明显差异,这些差异直接影响了种植体的稳定性和使用寿命,见表1。 钛具有更高的抗拉强度,表明钛与氧化锆相比具有出色的机械强度。BOJKO等[36]的一项研究评估了钛合金在轴向拉伸实验中的静态强度,突出了其高极限拉伸强度。氧化锆具有较高的弹性模量,表明其具有增强的抗变形潜力。?ZDO?AN等[37]发现研磨可使氧化锆陶瓷产生最高的硬度值,强调其抗压痕和磨损能力。另外,钛表现出优异的耐久性,抗疲劳性优于氧化锆。PETER等[38]的研究显示,经过最先进的疲劳实验机循环负载后,与氧化锆和陶瓷相比,钛的抗疲劳性更优越。氧化锆表现出更高的硬度,强调了其抗压痕和磨损性能。ZIDAN等[39]的研究显示,与含有硅烷化氧化锆的纳米复合材料组相比,含有氧化锆纳米粒子的聚甲基丙烯酸甲酯表面硬度显著提高。另有研究证实,添加10%,20%氧化锆纳米粒子显著提高了3D打印树脂的抗弯强度,并且生物相容性测试显示材料表面的细胞存活率大于80%[40]。TIKHILOV等[41]的研究证实,在抗拉强度为148 N的情况下,多孔钛植入物可实现与骨骼牢固附着的可能性,凸显了通过创新设计和处理进一步改善钛材料机械性能的潜力。 总之,虽然钛表现出优异的抗拉强度和抗疲劳性,但氧化锆表现出高硬度和弹性模量,这些性能可以通过表面处理和强化进一步增强,从而优化这些材料的机械性能。在选择种植体材料时,医生需要根据患者的具体情况和口腔环境进行综合考虑,以选择最适合的种植体材料。"

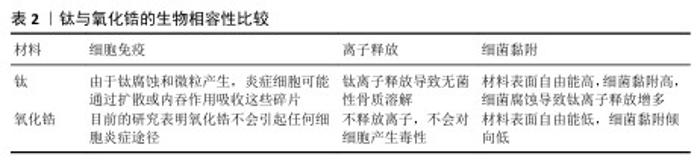
2.3 钛与氧化锆的生物相容性比较 氧化锆与钛种植体在生物相容性方面表现出显著差异,见表2,这对于种植体的成功植入及其长期稳定性至关重要。生物相容性通常涉及种植体与周围组织的相互作用,包括组织反应、炎症反应等生物学特性。 由于氧化锆对成骨细胞没有免疫抑制细胞毒性作用,细胞可以通过合成各种必需和结构蛋白质来加工细胞外基质[8]。根据SCHNURR等[42]的研究,氧化锆不会引起任何炎症反应。氧化锆具有良好生物相容性,因为不具有假致畸作用[43]。体内生物相容性研究显示,氧化锆在植入软组织后会形成一层薄涂层,与氧化铝中的纤维组织层相同[44]。氧化锆合成产品也不会在软组织中引发任何细胞毒性。一项研究制备了多孔Al2O3/ZrO2纳米复合材料(支架),结果显示Al2O3/ZrO2支架在骨组织工程中具有重要意义,可增强细胞增殖和黏附[45]。DE OLIVEIRA等[46]指出虽然氧化锆具有较低的表面自由能,但仍有致病菌黏附特性,只是细菌黏附倾向可能更低,这种良好的生物相容性使得氧化锆种植体在美学要求较高的区域(如前牙区)具有显著优势。氧化锆种植体的低过敏性和无毒性也使它在口腔种植领域得到广泛应用[47]。 相比之下,钛种植体植入后由于细菌腐蚀、表面磨损等原因导致金属离子释放,二氧化钛颗粒会影响许多细胞成分,进而引起免疫调节和炎症。当检测到外来损伤时,人体预期会产生先天细胞反应,Toll样受体介导细胞信号诱导炎症和适应性反应。在钛纳米颗粒的影响下,无论是否受到脂多糖刺激,Toll样受体4均会上调,最终增加肿瘤坏死因子α、白细胞介素1β和白细胞介素6的mRNA水 平[48],这些增加的炎症因子导致种植体周围组织损伤,发生无菌性骨溶解,引发骨丢失[49-50]。口腔最常见的细菌之一是变形链球菌,与龋齿及种植体表面腐蚀增加有关,其产生的致酸环境和脂多糖通过诱导电化学事件以及触发免疫细胞反应导致氧化攻击,对生物惰性钛表面产生负面影响,将金属离子引入种植体周围组织[51]。为增强钛的生物相容性,TANI等[52]将氮化硅沉积在纯钛金属表面,提高了材料表面的亲水性,增强了材料表面大鼠骨髓细胞的初始黏附,促进了硬组织分化,还表现出显著的抗菌活性。另外,在钛及钛合金表面涂覆天然生物材料(如生物蛋白、生长因子等)可显著改善材料表面的生物相容性[53]。由于这些天然生物材料表面具有天然的细胞识别位点,更有利于细胞与材料相互作用,例如,骨形态发生蛋白是一类具有相似结构、高度保守的功能蛋白,可刺激DNA合成和细胞复制,从而促进间充质细胞定向分化为成骨细胞,诱导骨和软骨形成[54]。 值得注意的是,尽管氧化锆和钛种植体在生物相容性方面均表现出色,但个体差异可能导致不同的反应,例如,某些患者可能对钛种植体产生过敏反应,而氧化锆种植体则通常不会引发此类问题。因此,在选择种植体材料时,医生需要根据患者的具体情况综合考虑。 "

2.4 钛与氧化锆的表面处理技术比较 氧化锆和钛种植体的表面处理技术在其临床应用效果中扮演着至关重要的角色,这些技术不仅影响着种植体的初期稳定性,还直接关系到种植体与周围骨组织的整合程度及长期种植成功率,见表3。 氧化锆种植体影响骨整合的因素可能与种植体设计和表面处理直接相关,包括表面形貌、粗糙度和润湿性[55]。目前,人们也非常重视氧化锆植入物表面的改进和开发,尤其是使其更加粗糙和可操作。通过化学、物理和热机械手段可以成功增强细胞反应,从而增强细胞的功能和对植入物表面的反应[56]。喷砂和酸蚀最初用于钛种植体以增加骨整合的表面积,氧化锆种植体目前也可采用类似工 艺[57],可获得理想的粗糙度、润湿性。酸蚀可显著抑制血链球菌和牙龈卟啉单胞菌生物膜的成熟[58-59]。另外,激光亦可使氧化锆的湿润性和粗糙度增加[60]。与钛类似,氧化锆的表面性质也可以通过施加各种涂层来改变,这些涂层可能包括二氧化硅、镁、氮、碳、羟基磷灰石、磷酸钙和多巴胺,进而改善氧化锆的生物特性[61-62]。 目前,钛种植体表面改性技术已发展出多种多样,这些技术大致可分为两类:第一种是表面处理,如喷砂[63]、酸蚀[64]、阳极氧化[65]、激光照射[66],这些技术有效地改变了钛的粗糙度、硬度和氧化性;另一类是涂层,在钛表面覆盖另一种材料,可以更大程度地改变植入物表面成分,实现更优异的生物医学功能。涂层技术包括等离子喷涂[67]、纳米喷雾干燥[68]、溶胶-凝胶法[69]、水热法[70]、自组装法[71]、3D打印等[72]。表面改性可改善钛种植体的机械性能、骨整合能力及抗菌性能等,甚至还能满足更丰富的定制化需求,如药物缓释等[73],显著提高种植成功率。 "


2.5 钛与氧化锆的骨整合能力比较 骨整合是影响种植牙成功的关键因素,氧化锆植入物的骨整合能力由多种因素决定,主要取决于表面粗糙度、能量、亲水性和生物反应[74]。XIUBING等[75]认为微纳米级表面设计是一种有效的表面改性方法,可以提高种植体的润湿性和摩擦性能。大多数人认为更粗糙的表面可提供更好的骨整合特性和更高的骨锚固潜力,然而,有研究表明过度的表面处理会导致表面粗糙度过高,从而产生微裂纹和缺陷,导致 材料的机械性能下降[76],因此,获得最佳表面粗糙度非常重要。MOURA等[77]认为亲水性与增强植入物的表面能密切相关。亲水性可通过材料表面润湿性程度来衡量,而材料表面润湿性与表面粗糙度有直接关系[78]。研究表明,亲水表面可使成骨细胞表型分化程度更高、骨形成程度和速率增加,并且整体成骨细胞附着得到改善[35]。 钛植入物表面会影响蛋白质吸附、血小板黏附、止血、炎症和成骨细胞反应的底层机制[79]。细胞对生物材料反应的主要初始阶段之一是细胞与植入物表面的连接[80]。附着受体(如整合素)通过蛋白质层介导细胞与细胞之间的连接,当植入物精确地定位在骨骼内部时,多种自然、物理、合成、加热和其他因素会影响骨整合发生。种植术后4周内,种植体表面可以明显看到新骨的发育,新骨与宿主骨上已经发育的骨骼相连(远距离成骨);在8-12周之间的某个时间点,种植体周围边界完全被成熟的层状骨取代,与种植体表面直接相连,从而结束骨整合[81]。由于出色的生物相容性、高品质和断裂强度,钛及其化合物已被广泛应用于承重情况下的牙科和骨科植入物[82]。Ti-6Al-4V植入物自1981年左右以来就一直用于制造牙科植入物,10年的临床种植成功率高达 99%,与骨和牙龈组织具有生物相容性[83]。ROEHLING等[84]通过检索相关文献发现,氧化锆和钛植入物显示出类似的软组织和硬组织整合能力,然而,与氧化锆植入物相比,钛植入物往往显示出更快的初始骨整合过程。PIERALLI等[85]的荟萃分析发现,钛种植体植入后与骨的接触面积大于氧化锆种植体。REMíSIO等[86]通过检索相关文献发现,钛种植体、氧化锆种植体植入后2个月与骨的接触面积比较无明显差异,而在植入后1,3个月与骨的接触面积出现显著差异。 "

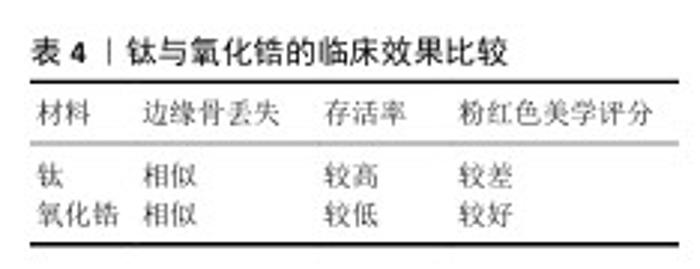
2.6 钛与氧化锆的临床效果比较 临床效果是评价种植牙是否成功的首要标准,主要体现在成功率、长期稳定性及患者满意度等方面。钛与氧化锆的临床效果比较见表4。 研究显示,氧化锆植入物10年后累积存活率估计值为 95.1%(总共4 017个植入物,2 083例患者),在失败的植入物中,植入物断裂发生率为 15.1%(172个植入物失败中有26个植入物断裂)[87],26个断裂种植体占纳入研究氧化锆种植体总数的0.65%,与一项涉及10 000多颗钛种植体研究观察到的0.44%断裂率相似[88]。断裂可能与种植体的直径有关,因为相当多的断裂发生在直径较小的种植体中。一项关于氧化锆种植体抗断裂性的体外研究结果表明,直径较小的氧化锆种植体(3.0-3.3 mm)在断裂时的弯矩比直径规则的种植体(3.8-4.4 mm)和直径较大的种植体(4.5-5.0 mm)低得多[31]。另外,氧化钇稳定化四方氧化锆陶瓷的稳定性在临床条件下可能会存在问题,因为材料会在化学活性水环境中长时间暴露于热应力和循环机械应力[89]。有研究显示,在长达132个月的长期观察中,氧化锆终种植体平均边缘骨丢失保持在0.632-2.060 mm之间,显示出与钛合金种植体相似的边缘骨丢失结果[90]。不同时间点边缘骨丢失平均值的波动可能是由于不同随访中的样本量差异及种植体配置不同[91]。 HAIMOV等[92]对301例患者的637个植入物进行评估,发现钛种植体的存活率高于氧化锆种植体(97.7%和93.8%)。FERNANDES等[93]研究显示,随访12-80个月后,氧化锆种植体的存活率为87.5%-91.25%,而钛种植体的存活率为92.6%-100%。 该两项研究的比较统计结果显示钛种植体更胜一筹。使用两件式锆种植体进行单区域修复或最多3个单元的修复研究显示,在30个月的随访中,氧化锆种植体的存活率为85.7%,而钛种植体的存活率为93.3%;在80个月的随访中,钛种植体的存活率仍然优于锆种植体[94]。有研究显示氧化锆与钛种植体的骨整合率相似[86]。对于粉红色美学评分,PADHYE等[44]认为氧化锆种植体的分值较好。KOLLER等[94]在使用两件式锆种植体修复时获得了最佳结果,其中氧化锆种植体的评分为10.33,而钛种植体的评分为9.0。THOMA等[95]指出颊侧软组织厚度约为 1.68 mm,氧化锆与钛牙种植体均存在黏膜变色,其中钛种植体组变色更明显[96]。这些差异在修复具有局部骨质流失或薄牙龈生物型等加重因素的美学部位时尤其重要。以上关于两种材料种植体的对比研究均存在样本量小、随访时间短的问题,对比结果仍需等待更大样本量以及更长随访时间的研究验证。 "

| [1] KRAMARCZYK K, SKOWRON K, SKOWRON P, et al. The multifaceted impact of missing teeth on general health: A narrative review. Folia Med. Cracov. 2024;64(1):25-37. [2] DEMIR E, ÖZEL G, İNAN Ö, et al. Analysis of Satisfaction Levels in Completely Edentulous Patients Treated with Different Configurations of Implant- Supported Prostheses. Int J Oral Maxillofac Implants. 2024;39(5):776-782. [3] SODNOM-ISH B, EO MY, KIM MJ, et al. A 10-year survival rate of tapered self-tapping bone-level implants from medically compromised Korean patients at a maxillofacial surgical unit. Maxillofac Plast Reconstr Surg. 2023;45(1):35. [4] VAGHELA H, EATON K. Is Zirconia a Viable Alternative to Titanium for Dental Implantology? Eur. J. Prosthodont Restor Dent. 2022;30(1):1-13. [5] HE X, REICHL FX, MILZ S, et al. Titanium and zirconium release from titanium- and zirconia implants in mini pig maxillae and their toxicity in vitro. Dent Mater. 2020;36(3):402-412. [6] BIHN SK, SON K, SON YT, et al. In Vitro Biofilm Formation on Zirconia Implant Surfaces Treated with Femtosecond and Nanosecond Lasers. J Funct Biomater. 2023;14(10):486. [7] WU H, CHEN X, KONG L, et al. Mechanical and Biological Properties of Titanium and Its Alloys for Oral Implant with Preparation Techniques: A Review. Materials (Basel). 2023;16(21):6860. [8] RAMCHARAN DN, ALAIMO KL, TIESENGA F. Diagnosis and Management of a Hypersensitivity Reaction to Titanium-Containing Surgical Clips: A Case Report. Cureus. 2023;15(2):e34929. [9] FIORILLO L, CICCIÙ M, TOZUM TF, et al. Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review. Materials (Basel). 2022;15(5):1979. [10] ZHAO S, ZHANG R, YU Q, et al. Cryoforged nanotwinned titanium with ultrahigh strength and ductility. Science. 2021;373(6561):1363-1368. [11] RAWAT P, SAXENA D, SHARMA A. Dr. Per-Ingvar Branemark: The Father of Modern Dental Implantology. Cureus. 2024;16(11): e73950. [12] PICCOLI C, SOLIANI G, PICCOLI P, et al. Long-Term Success in Dental Implant Revisions: A 31-Year Case Study of Alveolar Atrophy Management in an Elderly Woman. Am J Case Rep. 2024;6(25): e943341. [13] ALBREKTSSON T, WENNERBERG A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. 2019; 21 Suppl 1:4-7. [14] DE BRUYN H, RAES S, OSTMAN PO, et al. Immediate loading in partially and completely edentulous jaws: a review of the literature with clinical guidelines. Periodontol 2000. 2014;66(1):153-187. [15] WANG J, WANG B, LIU YY, et al. Recent Advances in Digital Technology in Implant Dentistry. J Dent Res. 2024;103(8):787-799. [16] RACOVITA AD. Titanium Dioxide: Structure, Impact, and Toxicity. Int J Environ Res Public Health. 2022;19(9):5681. [17] KUMARAGE GWC, HAKKOUM H, COMINI E. Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing. Nanomaterials (Basel). 2023;13(8):1424. [18] ROY M, CHELUCCI E, CORTI A, et al. Biocompatibility of Subperiosteal Dental Implants: Changes in the Expression of Osteogenesis-Related Genes in Osteoblasts Exposed to Differently Treated Titanium Surfaces. J Funct Biomater. 2024;15(6):146. [19] FLOREAN CT, CHIRA M, VERMEȘAN H, et al. The Influence of Using Recycled Waste Aggregates and Adding TiO(2) Nanoparticles on the Corrosion Resistance of Steel Reinforcement Embedded in Cementitious Composite. Materials (Basel). 2024;17(16):3895. [20] IBRAHIM MA, NASR GM, AHMED RM, et al. Physical characterization, biocompatibility, and antimicrobial activity of polyvinyl alcohol/sodium alginate blend doped with TiO2 nanoparticles for wound dressing applications. Sci Rep. 2024;14(1):5391. [21] ELLAKANY P, ALGHAMDI MA, ALSHEHRI T, et al. Cytotoxicity of Commercially Pure Titanium (cpTi), Silver-Palladium (Ag-Pd), and Nickel-Chromium (Ni-Cr) Alloys Commonly Used in the Fabrication of Dental Prosthetic Restorations. Cureus. 2022;14(11):e31679. [22] TAKAHASHI M, SATO K, TOGAWA G, et al. Mechanical Properties of Ti-Nb-Cu Alloys for Dental Machining Applications. J Funct Biomater. 2022;13(4):263. [23] PRANDO D, BRENNA A, DIAMANTI MV, et al. Corrosion of titanium: Part 2: Effects of surface treatments. J Appl Biomater Funct Mater. 2018;16(1):3-13. [24] HANAWA T. Zirconia versus titanium in dentistry: A review. Dent Mater J. 2020; 39(1):24-36. [25] BAPAT RA, YANG HJ, CHAUBAL TV, et al. Review on synthesis, properties and multifarious therapeutic applications of nanostructured zirconia in dentistry. RSC Adv. 2022;12(20):12773-12793. [26] CHILE J, DOLORES A, ESPINOZA-CARHUANCHO F, et al. Zirconia Dental Implants as a Different Alternative to Titanium: A Literature Review. J Int Soc Prev Community Dent. 2023;13(5):357-364. [27] GUL A, PAPIA E, NAIMI-AKBAR A, et al. Zirconia dental implants; the relationship between design and clinical outcome: A systematic review. J Dent. 2024;143:104903. [28] KONGKIATKAMON S, ROKAYA D, KENGTANYAKICH S, et al. Current classification of zirconia in dentistry: an updated review. PeerJ. 2023;11:e15669. [29] WU T, ZHOU Q, HONG G, et al. A chlorogenic acid-chitosan complex bifunctional coating for improving osteogenesis differentiation and bactericidal properties of zirconia implants. Colloids Surf B Biointerfaces. 2023;230:113484. [30] FRANCESCATO O, SOUZA RODRIGUES IN, DOUGLAS DE OLIVEIRA DW, et al. Primary Stability and Fracture Resistance of Zirconia and Titanium Implants: A Paired Comparative In Vitro Study. Int J Oral Maxillofac Implants. 2024:1-22. doi: 10.11607/10974. [31] BETHKE A, PIERALLI S, KOHAL RJ, et al. Fracture Resistance of Zirconia Oral Implants In Vitro: A Systematic Review and Meta-Analysis. Materials (Basel). 2020;13(3):562. [32] CHIOU LL, PANARIELLO BHD, HAMADA Y, et al. Comparison of In Vitro Biofilm Formation on Titanium and Zirconia Implants. Biomed Res Int. 2023;2023:8728499. [33] MIURA S, SHINYA A, ISHIDA Y, et al. Mechanical and surface properties of additive manufactured zirconia under the different building directions. J Prosthodont Res. 2023;67(3):410-417. [34] BRAVO E, SERRANO B, RIBEIRO-VIDAL H, et al. Biofilm formation on dental implants with a hybrid surface microtopography: An in vitro study in a validated multispecies dynamic biofilm model. Clin. Oral Implants Res. 2023;34(5):475-485. [35] MAJHI R, MAJHI RK, GARHNAYAK L, et al. Comparative evaluation of surface-modified zirconia for the growth of bone cells and early osseointegration. J Prosthet Dent. 2021;126(1):92.e91-92.e98. [36] BOJKO Ł, RYNIEWICZ AM, RYNIEWICZ W. Strength Tests of Alloys for Fixed Structures in Dental Prosthetics. Materials (Basel). 2022;15(10):3497. [37] OZDOGAN A, YESIL DUYMUS Z. Investigating the Effect of Different Surface Treatments on Vickers Hardness and Flexural Strength of Zirconium and Lithium Disilicate Ceramics. J Prosthodont. 2020;29(2):129-135. [38] PETER C, SHAH K, SIMON L, et al. Comprehensive Evaluation of Titanium, Zirconia, and Ceramic Dental Implant Materials: A Comparative Analysis of Mechanical and Esthetic Properties. Cureus. 2024;16(5):e60582. [39] ZIDAN S, SILIKAS N, AL-NASRAWI S, et al. Chemical Characterisation of Silanised Zirconia Nanoparticles and Their Effects on the Properties of PMMA-Zirconia Nanocomposites. Materials (Basel). 2021; 14(12):3212. [40] ALSHAMRANI A, ALHOTAN A, KELLY E, et al. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers (Basel). 2023;15(11):2523. [41] TIKHILOV R, SHUBNYAKOV I, DENISOV A, et al. The experimental study of tissue integration into porous titanium implants. Hip Int. 2022;32(3):386-390. [42] SCHNURR E, VOLZ KU, MOSETTER K, et al. Interaction of Telomere Length and Inflammatory Biomarkers Following Zirconia Implant Placement: A Case Series. J Oral Implantol. 2023;49(5):524-531. [43] SAITO MM, ONUMA K, YAMAKOSHI Y. Nanoscale osseointegration of zirconia evaluated from the interfacial structure between ceria-stabilized tetragonal zirconia and cell-induced hydroxyapatite. J Oral Biosci. 2024;66(2):281-287. [44] PADHYE NM, CALCIOLARI E, ZUERCHER AN, et al. Survival and success of zirconia compared with titanium implants: a systematic review and meta-analysis. Clin. Oral Investig. 2023;27(11): 6279-6290. [45] VIDANE AS, NUNES FC, FERREIRA JA, et al. Biocompatibility and interaction of porous alumina-zirconia scaffolds with adipose-derived mesenchymal stem cells for bone tissue regeneration. Heliyon. 2023;9(9):e20128. [46] DE OLIVEIRA GR, POZZER L, CAVALIERI-PEREIRA L, et al. Retraction: Bacterial adhesion and colonization differences between zirconia and titanium implant abutments: an in vivo human study. J Periodontal Implant Sci. 2019;49(1):58. [47] SAINI RS, MOSADDAD SA, HEBOYAN A. Application of density functional theory for evaluating the mechanical properties and structural stability of dental implant materials. BMC Oral Health. 2023;23(1):958. [48] NOUMBISSI S, SCARANO A, GUPTA S. A Literature Review Study on Atomic Ions Dissolution of Titanium and Its Alloys in Implant Dentistry. Materials (Basel). 2019; 12(3):368. [49] ZHOU Z, SHI Q, WANG J, et al. The unfavorable role of titanium particles released from dental implants. Nanotheranostics. 2021;5(3):321-332. [50] WANG X, LI Y, FENG Y, et al. Macrophage polarization in aseptic bone resorption around dental implants induced by Ti particles in a murine model. J Periodontal Res. 2019;54(4):329-338. [51] BERRYMAN Z, BRIDGER L, HUSSAINI HM, et al. Titanium particles: An emerging risk factor for peri-implant bone loss. Saudi Dent J. 2020;32(6):283-292. [52] TANI A, TSUBOUCHI H, MA L, et al. Effect of Silicon Nitride Coating on Titanium Surface: Biocompatibility and Antibacterial Properties. Int J Mol Sci. 2024;25(17):9148. [53] CHE Z, SUN Q, ZHAO Z, et al. Growth factor-functionalized titanium implants for enhanced bone regeneration: A review. Int J Biol Macromol. 2024;274(Pt 2):133153. [54] HAN JJ, YANG HJ, HWANG SJ. Enhanced Bone Regeneration by Bone Morphogenetic Protein-2 after Pretreatment with Low-Intensity Pulsed Ultrasound in Distraction Osteogenesis. Tissue Eng Regen Med. 2022; 19(4):871-886. [55] CRUZ MB, SILVA N, MARQUES JF, et al. Biomimetic Implant Surfaces and Their Role in Biological Integration-A Concise Review. Biomimetics (Basel). 2022;7(2):74. [56] HO KN, CHEN LW, KUO TF, et al. Surface modification of zirconia ceramics through cold plasma treatment and the graft polymerization of biomolecules. J Dent Sci. 2023;18(1):73-80. [57] CHOI SH, RYU JH, KWON JS, et al. Effect of wet storage on the bioactivity of ultraviolet light- and non-thermal atmospheric pressure plasma-treated titanium and zirconia implant surfaces. Mater Sci Eng C Mater Biol Appl. 2019;105:110049. [58] KLIGMAN S, REN Z, CHUNG CH, et al. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J Clin Med. 2021;10(8): 1641. [59] LIAO M, SHI Y, CHEN E, et al. The Bio-Aging of Biofilms on Behalf of Various Oral Status on Different Titanium Implant Materials. Int J Mol Sci. 2022;24(1):332. [60] FERNANDES BF, SILVA N, DA CRUZ MB, et al. Cell Biological and Antibacterial Evaluation of a New Approach to Zirconia Implant Surfaces Modified with MTA. Biomimetics (Basel). 2024;9(3):155. [61] SCHüNEMANN FH, GALÁRRAGA-VINUEZA ME, MAGINI R, et al. Zirconia surface modifications for implant dentistry. Mater Sc Eng C Mater Biol Appl. 2019;98: 1294-1305. [62] LI X, LIANG S, INOKOSHI M, et al. Different surface treatments and adhesive monomers for zirconia-resin bonds: A systematic review and network meta-analysis. Jpn Dent Sci Rev. 2024;60:175-189. [63] YANG R, HONG MH. Improved Biocompatibility and Osseointegration of Nanostructured Calcium-Incorporated Titanium Implant Surface Treatment (XPEED(®)). Materials (Basel). 2024;17(11): 2707. [64] BAYRAK M, KOCAK-OZTUG NA, GULATI K, et al. Influence of Clinical Decontamination Techniques on the Surface Characteristics of SLA Titanium Implant. Nanomaterials (Basel). 2022;12(24):4481. [65] GULATI K, ZHANG Y, DI P, et al. Research to Clinics: Clinical Translation Considerations for Anodized Nano-Engineered Titanium Implants. ACS Biomater Sci Eng. 2022;8(10): 4077-4091. [66] VEIKO V, KARLAGINA Y, ZERNITCKAIA E, et al. Laser-Induced µ-Rooms for Osteocytes on Implant Surface: An In Vivo Study. Nanomaterials (Basel). 2022;12(23):4229. [67] BHATTACHARJEE A, BANDYOPADHYAY A, BOSE S. Plasma sprayed fluoride and zinc doped hydroxyapatite coated titanium for load-bearing implants. Surf Coat Technol. 2022;440:128464. [68] BAGHDAN E, RASCHPICHLER M, LUTFI W, et al. Nano spray dried antibacterial coatings for dental implants. Eur J Pharm Biopharm. 2019;139:59-67. [69] AZARI R, REZAIE HR, KHAVANDI A. Effect of titanium dioxide intermediate layer on scratch and corrosion resistance of sol-gel-derived HA coating applied on Ti-6Al-4V substrate. Prog Biomater. 2021;10(4): 259-269. [70] FU Z, DENG X, FANG X. Effect of addition of Ca2+ to titanium by a hydrothermal method on soft tissue sealing. Microsc Res Tech. 2022;85(9):3050-3055. [71] CHEN H, FENG R, XIA T, et al. Progress in Surface Modification of Titanium Implants by Hydrogel Coatings. Gels. 2023;9(5):423. [72] CHENG XQ, XU W, SHAO LH, et al. Enhanced osseointegration and antimicrobial properties of 3D-Printed porous titanium alloys with copper-strontium doped calcium silicate coatings. J Biomater Appl. 2025;39(6):607-619. [73] ZHAN J, LI L, YAO L, et al. Evaluation of sustained drug release performance and osteoinduction of magnetron-sputtered tantalum-coated titanium dioxide nanotubes. RSC Adv. 2024;14(6): 3698-3711. [74] RAUSCH MA, SHOKOOHI-TABRIZI H, WEHNER C, et al. Impact of Implant Surface Material and Microscale Roughness on the Initial Attachment and Proliferation of Primary Human Gingival Fibroblasts. Biology (Basel). 2021;10(5):356. [75] XIUBING J, QILEI Z, DU Z, et al. Wettability and frictional properties on zirconia surfaces irradiated by femtosecond laser. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2022;654:130198 [76] KIM DS, LEE JK. Sintered Characteristics of 3 Mole% Yttria-Stabilized Zirconia Polycrystals (3Y-TZP) Implants Manufactured by Slip-Casting and Computer Aided Design/Computer Aided Manufacturing (CAD/CAM). J Nanosci Nanotechnol. 2021;21(7): 3877-3881. [77] MOURA CG, PEREIRA R, BUCIUMEANU M, et al. Effect of laser surface texturing on primary stability and surface properties of zirconia implants. Ceram Int. 2017;43(17):15227-15236. [78] ARAGONESES J, VALVERDE NL, FERNANDEZ-DOMINGUEZ M, et al. Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors. Materials (Basel). 2022;15(11): 4036. [79] WU B, TANG Y, WANG K, et al. Nanostructured Titanium Implant Surface Facilitating Osseointegration from Protein Adsorption to Osteogenesis: The Example of TiO2 NTAs. Int J Nanomedicine. 2022;17: 1865-1879. [80] FANG X, SUN D, LI Y, et al. Macrophages in the process of osseointegration around the implant and their regulatory strategies. Connect. Tissue Res. 2024;65(1):1-15. [81] WANG S, ZHAO X, HSU Y, et al. Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta Biomater. 2023;169: 19-44. [82] KHAOHOEN A, SORNSUWAN T, CHAIJAREENONT P, et al. Biomaterials and Clinical Application of Dental Implants in Relation to Bone Density-A Narrative Review. J Clin Med. 2023;12(21):6924. [83] NICHOLSON JW. Titanium Alloys for Dental Implants: A Review. Prosthesis. 2020;2(2): 100. [84] ROEHLING S, SCHLEGEL KA, WOELFLER H, et al. Zirconia compared to titanium dental implants in preclinical studies-A systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(5):365-395. [85] PIERALLI S, KOHAL RJ, LOPEZ HERNANDEZ E, et al. Osseointegration of zirconia dental implants in animal investigations: A systematic review and meta-analysis. Dent Mater. 2018;34(2):171-182. [86] REMíSIO M, BORGES T, CASTRO F, et al. Histologic Osseointegration Level Comparing Titanium and Zirconia Dental Implants: Meta-analysis of Preclinical Studies. Int J Oral Maxillofac Implants. 2023; 38(4):667-680. [87] MOHSENI P, SOUFI A, CHRCANOVIC BR. Clinical outcomes of zirconia implants: a systematic review and meta-analysis. Clin Oral Investig. 2023;28(1):15. [88] CHRCANOVIC BR, KISCH J, ALBREKTSSON T, et al. Factors influencing the fracture of dental implants. Clin Implant Dent Relat Res. 2018;20(1):58-67. [89] ALFRISANY NM, DE SOUZA GM. Surface and bulk properties of zirconia as a function of composition and aging. J Mech Behav Biomed Mater. 2022;126:104994. [90] CHRCANOVIC BR, KISCH J, ALBREKTSSON T, et al. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin Implant Dent Relat Res. 2018;20(2):199-207. [91] WENNERBERG A, ALBREKTSSON T, CHRCANOVIC B. Long-term clinical outcome of implants with different surface modifications. Eur J Oral Implantol. 2018; 11 Suppl 1:S123-s136. [92] HAIMOV E, SARIKOV R, HAIMOV H, et al. Differences in Titanium, Titanium-Zirconium, Zirconia Implants Treatment Outcomes: a Systematic Literature Review and Meta-Analysis. J Oral Maxillofac Res. 2023;14(3):e1. [93] FERNANDES PRE, OTERO AIP, FERNANDES JCH, et al. Clinical Performance Comparing Titanium and Titanium-Zirconium or Zirconia Dental Implants: A Systematic Review of Randomized Controlled Trials. Dent J (Basel). 2022;10(5):83. [94] KOLLER M, STEYER E, THEISEN K, et al. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin Oral Implants Res. 2020;31(4):388-396. [95] THOMA DS, IOANNIDIS A, CATHOMEN E, et al. Discoloration of the Peri-implant Mucosa Caused by Zirconia and Titanium Implants. Int J Periodontics Restorative Dent. 2016;36(1):39-45. [96] RUIZ HENAO PA, CANEIRO QUEIJA L, MAREQUE S, et al. Titanium vs ceramic single dental implants in the anterior maxilla: A 12-month randomized clinical trial. Clin Oral Implants Res. 2021;32(8): 951-961. [97] II S. Quantitative Characterization by Transmission Electron Microscopy and Its Application to Interfacial Phenomena in Crystalline Materials. Materials (Basel). 2024;17(3):578. [98] BANNUNAH AM. Biomedical Applications of Zirconia-Based Nanomaterials: Challenges and Future Perspectives. Molecules. 2023; 28(14):5428. [99] HU J, ATSUTA I, LUO Y, et al. Promotional Effect and Molecular Mechanism of Synthesized Zinc Oxide Nanocrystal on Zirconia Abutment Surface for Soft Tissue Sealing. J Dent Res. 2023;102(5):505-513. [100] MOHAMMED MK, ALAHMARI A, ALKHALEFAH H, et al. Evaluation of zirconia ceramics fabricated through DLP 3d printing process for dental applications. Heliyon. 2024;10(17):e36725. [101] GRADIȘTEANU-PIRCALABIORU G, NEGUT I, DINU M, et al. Enhancing orthopaedic implant efficacy: the development of cerium-doped bioactive glass and polyvinylpyrrolidone composite coatings via MAPLE technique. Biomed Mater. 2024;20(1). doi:10.1088/1748-605X/ad98d5. [102] SHARMA SK, GAJEVIĆ S, SHARMA LK, et al. Magnesium-Titanium Alloys: A Promising Solution for Biodegradable Biomedical Implants. Materials (Basel). 2024;17(21):5157. |
| [1] | Chen Haojie, Wang Dai, Shen Shan. Immune inflammatory microenvironment mechanisms in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2054-2062. |
| [2] | Zheng Xuying, Hu Hongcheng, Xu Libing, Han Jianmin, Di Ping. Stress magnitude and distribution in two-piece cement-retained zirconia implants under different loading conditions and with varying internal connection shapes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1979-1987. |
| [3] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [4] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [5] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [6] | Li Ruiqiang, Yin Chen, Ma Yan. Effect of carbamide peroxide and hydrogen peroxide bleaching agents on laser-induced fluorescence in dentin Raman spectroscopy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 296-302. |
| [7] | Zhao Chunhong, He Li. Comparison of effects of three machined nitinol instruments on preparing curved root canals using different methods [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 303-309. |
| [8] | Cheng Yanan, Yu Jiazhi, Liu Yinchang, Wu Jie, Yu Tong, Wang Lu, Li Xiaoguang. Three-dimensional finite element analysis of molar distalization with clear aligners with different thicknesses and edges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 310-318. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||