Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (8): 2072-2080.doi: 10.12307/2026.602
Previous Articles Next Articles
Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy
Liu Yang1, Liu Donghui1, Xu Lei1, Zhan Xu1, Sun Haobo1, Kang Kai1, 2, 3
- 1Department of Cardiovascular Surgery, 2NHC Key Laboratory of Cell Transplantation, 3Key Laboratory of Hepatosplenic Surgery of Ministry of Education, The First Affiliated Hospital of Harbin Medical University, Harbin 150000, Heilongjiang Province, China
-
Received:2025-01-21Accepted:2025-03-24Online:2026-03-18Published:2025-07-28 -
Contact:Kang Kai, Professor, Chief physician, Department of Cardiovascular Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin 150000, Heilongjiang Province, China; NHC Key Laboratory of Cell Transplantation, The First Affiliated Hospital of Harbin Medical University, Harbin 150000, Heilongjiang Province, China; Key Laboratory of Hepatosplenic Surgery of Ministry of Education, The First Affiliated Hospital of Harbin Medical University, Harbin 150000, Heilongjiang Province, China -
About author:Liu Yang, Master candidate, Physician, Department of Cardiovascular Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin 150000, Heilongjiang Province, China Liu Donghui, Doctoral candidate, Physician, Department of Cardiovascular Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin 150000, Heilongjiang Province, China -
Supported by:National Natural Science Foundation of China, No. 82472147 (to KK); Key Research and Development Program of Heilongjiang Province, No. 2023ZX06C04 (to KK); Open Fund of Key Laboratory of Hepatosplenic Surgery, Ministry of Education, China, No. GPKF202402 (to KK)
CLC Number:
Cite this article
Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

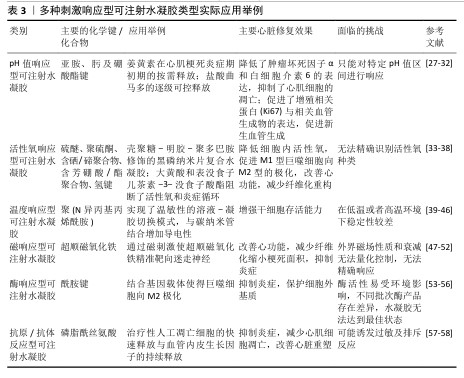
2.2 pH值响应型可注射水凝胶 在急性心肌梗死的早期,心肌细胞由于缺血缺氧导致能量代谢方式向无氧酵解转换,梗死区域局部乳酸、丙酮酸等代谢产物堆积,导致局部pH值明显降低。亚胺、肟和硼酸酯键等的动态化学键通常对局部酸性环境反应敏感,故常被作为目标化学键用以合成相应的pH值响应型水凝胶[27]。 由于独特的理化性质,pH值响应水凝胶能够作为有效的药物载体。药物可以通过物理吸附或化学共价结合的方式加载到水凝胶中,当水凝胶在目标部位的pH值条件下降解时药物得以释放,这种机制使得pH值响应水凝胶在心肌梗死等局部炎症环境中表现出良好的释放特性,可以实现药物的定向输送和持续释放[28-30]。总体来说,pH值响应型可注射水凝胶不仅为梗死区域提供物理支撑,同时可以装载相关治疗物质防止炎症持续进展,防止心肌细胞凋亡和坏死等病理生理进程。然而,pH值响应型可注射水凝胶只能对特定的pH值区间进行效果显著响应,这可能对水凝胶的体内应用有一定限制作用。 在心肌梗死的初始炎症阶段,姜黄素等小分子抗炎药物已被证实是有效的抗炎药物。重组人胶原蛋白Ⅲ结构与天然胶原蛋白相似,具有良好的细胞相容性和低免疫原性,可促进新生血管生成,是构建水凝胶的有效基质材料。基于联合治疗的理念,HU等[31]通过在羧甲基纤维素-苯硼酸聚合物中引入3-氨基苯硼酸和聚乙烯醇形成具有pH值响应性的硼酸酯键,再将聚乳酸-羟基乙酸共聚物纳米颗粒包裹的姜黄素和重组人胶原蛋白Ⅲ组合装入水凝胶,结果表明,在心肌梗死后的酸性环境中,姜黄素因水凝胶的降解按需释放,这有效降低了肿瘤坏死因子α和白细胞介素6的表达、抑制了心肌细胞的凋亡,有效促进了增殖相关蛋白(Ki67)和相关血管生成物的表达,促进新生血管生成。 NOUREEN等[32]利用天然聚合物杏仁胶和合成单体丙烯酸,以N,N-亚甲基双丙烯酰胺作为交联剂,通过自由基聚合方法制备的杏仁胶-co-丙烯酸水凝胶表现出良好的pH值响应性,其中羧酸基团(-COOH)和氢键在药物释放过程中起关键作用,同时杏仁胶和丙烯酸的比例增加会增加释药行为,N,N-亚甲基双丙烯酰胺比例的增加则会降低释药行为。以具有抗炎、止痛作用的盐酸曲马多为模型药物,该水凝胶能够在不同pH值实验环境变化下实现药物逐级可控释放特性;药代动力学评价证实,与药物的口服溶液相比,该水凝胶具有将实验药物的生物利用度维持较长时间的能力。这种新型水凝胶的开发为以后实现“精准+持续”心肌梗死治疗的理念奠定了基础。 "


2.3 活性氧响应型可注射水凝胶 心肌梗死后,心肌细胞缺氧导致心肌细胞线粒体能量代谢障碍,产生大量的活性氧,高活性氧会对蛋白质产生不良影响,损害细胞功能,导致细胞面临死亡的风险[33]。过量活性氧的产生与心肌梗死炎症期密切相关。活性氧反应性聚合物载体可以分为以下几大类:硫醚聚合物、聚硫酮聚合物、含硒/碲聚合物、含芳硼酸/酯聚合物、草酸芳酯和聚脯氨酸等,这些聚合物通过化学键断裂或者增加亲水性来实现载体药物释放[34]。同时,可以在水凝胶中添加活性氧清除剂去除活性氧,目前新型的活性氧清除剂类型主要包括硫醚基团[35]、金属离子配位、聚合物链段的设计[36],目前大多数活性氧响应型水凝胶的治疗目的是去除细胞外的活性氧。当载药凝胶到达病变位置时,荷载药物从水凝胶中释放出来中和活性氧离子,从而减轻活性氧诱发的炎性反应。但是,活性氧的种类不同,比如超氧阴离子、过氧化氢等,活性氧响应型可注射水凝胶面临无法准确识别活性氧种类导致响应难以控制的困境,这是需要解决的问题。 过量活性氧是导致炎症因子分泌的重要因素,而M2表型巨噬细胞起到抗炎作用。在既往研究中,传统水凝胶材料(如壳聚糖和明胶)已被广泛应用于组织工程,具有良好的生物安全性,但抗氧化能力有限。ZHANG等[37]尝试去解决有效清除过量活性氧并促进M2型巨噬细胞极化以改善心肌微环境的挑战,他们将壳聚糖和明胶结合,形成具有控制释放能力的水凝胶,随后将聚多巴胺修饰的黑磷纳米片封装在其中形成复合水凝胶,通过左前降支冠状动脉结扎建立心肌梗死模型,实验结果显示该复合水凝胶显著降低了H9C2细胞内活性氧水平、提高了细胞活力,巨噬细胞表面标记物检测显示白细胞介素6表达显著降低、白细胞介素10表达显著提高,表明该水凝胶能够有效促进M1型巨噬细胞向M2型的极化。该复合水凝胶水凝胶在前7 d内释放速率较快,随后逐渐减缓,这种释放模式可以满足心肌梗死治疗过程中对药物的持续需求,确保在关键的治疗窗口期内药物能够持续发挥作用。综上所述,复合水凝在改善心肌梗死炎症微环境和促进巨噬细胞转化治疗心肌梗死方面具有协同作用。 在心肌细胞受损过程中,活性氧和炎症构成的恶性循环会不断加重心肌细胞受损,活性氧和Toll样受体4是这个循环的关键节点,因此,减少活性氧的产生和阻断Toll样受体4是治疗心肌梗死的可行方法。大黄素主要从中药大黄中分离出来,它可抑制Toll样受体4/核因子κB抗炎信号通路起到抗炎作用,但大黄素较差的稳定性和水溶性限制了其临床应用。表没食子儿茶素-3-没食子酸酯具有良好的活性氧清除能力。利用表没食子儿茶素-3-没食子酸酯与Rh凝胶之间的π-π堆积和氢键非共价相互作用,LIAO等[38]制备了表没食子儿茶素-3-没食子酸酯@Rh明胶可注射水凝胶系统,以实现Rh和表没食子儿茶素-3-没食子酸酯的局部精准释放,通过活性氧清除和Toll样受体4抑制有效阻断了活性氧炎症循环。在缺血再灌注损伤后,将表没食子儿茶素-3-没食子酸酯@Rh-明胶注射到小鼠心肌梗死边界区域后,该水凝胶实现了炎症和氧化应激的双重抑制,改善心肌功能,显著减少纤维化重构。这种多功能水凝胶的开发为心肌梗死的治疗提供了新的思路。 "


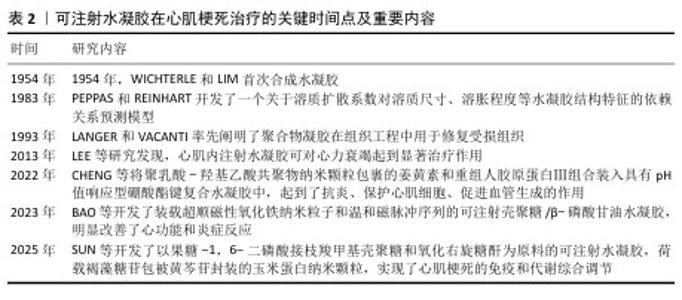
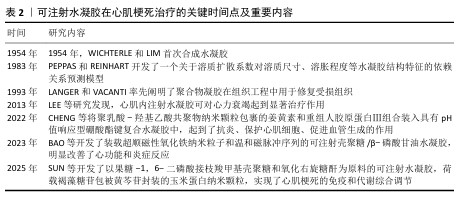
2.4 温度响应型可注射水凝胶 温度响应型水凝胶是对体内温度变化敏感的智能化聚合物凝胶,温度变化会影响这种水凝胶的亲水基团和疏水基团作用,从而导致水凝胶结构和功能发生变化[39]。聚(N-异丙基丙烯酰胺)是温敏水凝胶材料中应用最广泛的一种,其化学结构中含有1个疏水性异丙基和1个亲水性酰胺基,它的一个重要特征是低临界溶解温度,通常在约32 ℃。当温度低于临界溶解温度时,聚(N-异丙基丙烯酰胺)水凝胶呈现亲水性,能够吸收大量水分,形成透明的凝胶状态;而当温度超过临界溶解温度时,聚(N-异丙基丙烯酰胺)则转变为疏水性,释放水分,导致凝胶体积缩小,形成更为密实的结构[40-41]。同时,聚(N-乙烯基己内酰胺)材料可以通过调节其单体组成来实现温度响应性药物释放,由于它具有良好的生物相容性,在生物医学上显示了良好的应用前景[42]。根据相关研究报道,这种水凝胶可以直接注射入心肌内,在体温下迅速形成局部凝胶为受损心肌提供物理支撑,同时可作为治疗因子载体用于心肌梗死修复治疗[43-45]。然而,这些材料在低温或者高温环境下的稳定性较差,存在难以存储的问题,可能需要增加额外成分进行优化。 急性心肌梗死后细胞坏死,坏死的心肌细胞反过来会加重炎症级联反应,损害存活的心肌细胞,最终导致心力衰竭的发生,那么增加存活心肌细胞数量有必要的。单壁碳纳米管对电活性细胞(如心肌细胞和神经元)或组织具有积极影响。LI等[46]开发了一种新型的聚(N-异丙基丙烯酰胺)联合单壁碳纳米管基热敏水凝胶,此水凝胶显示了良好的温敏性,它可由31 ℃的液态转换为37 ℃的固态形式,实现了良好溶胶-凝胶切换模式,当用于心肌细胞培养时,碳纳米管修饰水凝胶在心肌细胞增殖和电活动方面表现出优越的生物活性;将该水凝胶联合干细胞移植修复心肌损伤时,干细胞的黏附和存活能力显著提高,这种特性使得这种水凝胶在促进心脏再生方面具有较大的开发潜力。 2.5 磁响应型可注射水凝胶 心肌梗死后死亡的心脏组织引起炎症反应系统失调[47],自主神经功能紊乱会加重炎症反应,而中枢神经系统促使迷走神经释放乙酰胆碱,乙酰胆碱作用于α7烟碱乙酰胆碱受体,该受体通过胆碱能抗炎通路实现梗死组织的抗炎作用[48]。电磁场可以调控心脏自主神经的结构和功能[49]。迷走神经刺激恢复自主神经稳态、减少全身系统炎症,而利用迷走神经刺激治疗过程中电极的植入面临感染风险[50]。磁性响应材料能够在没有直接接触的情况下进行外部磁场操控,减少侵入性操作所带来的问题[51]。但是,外界磁场的强度、频率等因素可能难以控制,同时磁场穿过人体时会形成不定量性的衰减,使得磁响应型可注射水凝胶无法达到最佳治疗效果,这是一个需要解决的问题。 BAO等[52]开发了装载超顺磁性氧化铁纳米粒子和温和磁脉冲序列的可注射壳聚糖/β-磷酸甘油水凝胶,此水凝胶实现了体内的微创植入,并且可以通过磁刺激将超顺磁性氧化铁纳米粒子精准靶向单个迷走神经,从而克服磁场聚焦性差的难题。在温和的磁场(约100 mT)下,原位注射该水凝胶可显著改善心肌梗死大鼠的心功能,明显减少纤维化、缩小梗死面积,同时抑制炎症细胞浸润和炎症因子表达。 "


2.6 酶响应型可注射水凝胶 酶响应型水凝胶是在水凝胶3D网络中载入酶识别单元,之后酶与识别单元特异性识别并结合产生相互作用,导致水凝胶特性发生改变,实现水凝胶的酶降解性,最终药物及相应治疗因子智能化释放[53-55]。然而,酶响应型可注射水凝胶必须在保证酶活性最佳的情况下进行设计,同时酶在不同环境下的活性有差异,不同批次的酶产品的品质存在差异,导致水凝胶无法持续达到最佳状态,这就需要投入更多的研发成本和精力去不断探索。 心肌梗死后微环境发生剧烈变化,尤其是基质金属蛋白酶(基质金属蛋白酶2/9)的过度表达。CHEN等[56]制备了新型响应性水凝胶系统(MPGC4),MPGC4由四聚乙烯醇水凝胶和白细胞介素4-pDNA复合碳点和基因载体(CTL4)组成,在合成过程中,氨基与醛基或羧基发生反应形成酰胺键(-CONH-),这也是水凝胶结构的重要组成部分,流变学特性显示此水凝胶能够在注射后适应心脏的剪切和振动变化,通过基质金属蛋白酶2/9的特异性识别触发CTL4的释放,该系统的设计使得CTL4能够有效地被巨噬细胞摄取而显著降低M1巨噬细胞标志物CD86的表达,显著增加M2标志物CD206的表达,减轻炎症反应;同时,此系统还抑制基质金属蛋白酶的表达以减少心脏细胞外基质的变性。MPGC4在体内具有良好的生物相容性,能够在心脏中稳定保留,避免不可控泄漏。 "

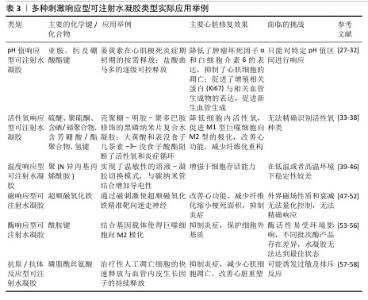
2.7 抗原/抗体反应型水凝胶 抗原或抗体可以通过化学偶联或聚合保留在水凝胶内部,也可以与大分子结合,在水凝胶基质内提供可逆交联[57]。由于3D结构水凝胶具有很高的亲和性和稳定性,使得水凝胶成为化学物质的良好载体。可注射水凝胶的降解或者释放需要由抗原或抗体来触发,目前还没有报道真正意义上抗原/抗体响应型可注射水凝胶,但是其他相关实验研究为开发这种水凝胶不断增添可能性。但是,抗原/抗体反应性水凝胶所负载的为外源性抗原或者抗体,在一些情况下可能会激活过敏及排斥反应,限制了其实际应用。 心肌梗死后炎症反应与心脏重塑之间存在矛盾。ZHANG等[58]开发了一种新型的人工凋亡细胞与血管内皮生长因子联合注射的水凝胶,水凝胶主要由改性透明质酸、醛基透明质酸和醛基硫酸软骨素合成,旨在通过靶向递送方式实现调节免疫反应和促进血管再生来减轻心肌梗死后的心脏重塑。人工凋亡细胞通过定制的脂质体制备,外层暴露磷脂酰丝氨酸,巨噬细胞对人工凋亡细胞的识别和吞噬作用源于人工凋亡细胞膜上磷脂酰丝氨酸与巨噬细胞上磷脂酰丝氨酸受体的特异性结合,从而促进M2型巨噬细胞的极化,降低M1型巨噬细胞的比例,抑制炎症反应;血管内皮生长因子的持续释放有助于血管再生,改善心肌缺血后的血流供应。通过设计具有不同电荷相互作用的水凝胶,实现了人工凋亡细胞的快速释放和血管内皮生长因子的持续释放,提供了一种精确的时空调控策略。实验结果表明,该系统在大鼠心肌梗死模型中有效抑制了炎症反应、减少了心肌细胞凋亡,促进了心脏重塑的改善,并展示了良好的生物相容性。这项研究展示了可注射水凝胶封装抗原/抗体的良好能力,为以后开发抗原/抗体可注射水凝胶提供了良好开端。 多种刺激响应型可注射水凝胶类型实际应用举例,见表3。 2.8 4D生物打印技术对刺激响应型可注射水凝胶的促进作用 首先,3D生物打印将活细胞、生物活性分子和生物材料结合在3D支架内,生成结构上具有功能的生物构建[59],心脏3D生物打印技术主要有基于挤压的生物打印、基于激光的生物打印、基于喷墨的生物打印等[60]。使用生物墨水打印的支架具有低免疫原性[61]。与传统基于支架的方法相比,3D生物打印可以更准确地模拟天然的静态结构,但是4D生物打印对于天然组织动态特性的模拟方面更具优势。4D生物打印拓展了时间上的第四维度,重点是它打印的植入物可持续适应身体生长,模拟对微环境刺激的动态响应,还具有形状记忆和自启动功能,同时4D打印很有希望创造出可编程、适应性更强的多功能结构产品。目前,4D生物打印心血管植入物在体外和动物实验中已经显示出良好效果,充分证明了其在心血管系统中应用的巨大潜力[62-64]。4D生物打印已经在多种刺激响应型水凝胶中得到应用,基于水凝胶的生物墨水是最常见的生物墨水类型,4D生物打印将传统的生物打印生物材料与刺激响应生物材料结合形成新型材料墨水,可以合成可控性强、与目标适应佳和生物相容性更好的刺激响应型复合水凝胶,其中海藻酸钠和明胶基墨水应用广泛。单一刺激响应型水凝胶并不能适应复杂的病理变化,而4D生物打印可以具有实现合成多刺激响应水凝胶的巨大潜力,这针对进行全方位、全程化治疗理念具有重大意义[65]。"
| [1] GLADKA MM, KOHELA A, MOLENAAR B, et al. Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nat Commun. 2021;12(1):84. [2] ALIZADEH G, GHOLIPOUR K, KAZEMI SHISHAVAN M, et al. Future of myocardial infarction mortality in Iran: a scenario-based study. J Health Popul Nutr. 2023;42(1):19. [3] ZHENG Z, TAN Y, LI Y, et al. Biotherapeutic-loaded injectable hydrogels as a synergistic strategy to support myocardial repair after myocardial infarction. J Control Release. 2021;335:216-236. [4] FRANGOGIANNIS NG. Pathophysiology of Myocardial Infarction. Compr Physiol. 2015; 5(4):1841-1875. [5] SEROPIAN IM, TOLDO S, VAN TASSELL BW, et al. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63(16):1593-1603. [6] NAHRENDORF M, PITTET MJ, SWIRSKI FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121(22): 2437-2445. [7] SWIRSKI FK, NAHRENDORF M. Macrophage-stem cell crosstalk after myocardial infarction. J Am Coll Cardiol. 2013;62(20):1902-1904. [8] BEJERANO T, ETZION S, ELYAGON S, et al. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018;18(9):5885-5891. [9] CHEN J, YANG J, LIU R, et al. Dual-targeting Theranostic System with Mimicking Apoptosis to Promote Myocardial Infarction Repair via Modulation of Macrophages. Theranostics. 2017;7(17):4149-4167. [10] DAI Y, SONG J, LI W, et al. RhoE Fine-Tunes Inflammatory Response in Myocardial Infarction. Circulation. 2019;139(9): 1185-1198. [11] NGUYEN PD, DE BAKKER DEM, BAKKERS J. Cardiac regenerative capacity: an evolutionary afterthought? Cell Mol Life Sci. 2021;78(12):5107-5122. [12] MORSINK M, SEVERINO P, LUNA-CERON E, et al. Effects of electrically conductive nano-biomaterials on regulating cardiomyocyte behavior for cardiac repair and regeneration. Acta Biomater. 2022;139: 141-156. [13] BORGES FTP, PAPAVASILIOU G, TEYMOUR F. Characterizing the Molecular Architecture of Hydrogels and Crosslinked Polymer Networks beyond Flory-Rehner-I. Theory. Biomacromolecules. 2020;21(12): 5104-5118. [14] KOETTING MC, PETERS JT, STEICHEN SD, et al. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1-49. [15] SLAUGHTER BV, KHURSHID SS, FISHER OZ, et al. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32-33):3307-3329. [16] BERTSCH P, DIBA M, MOONEY DJ, et al. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem Rev. 2023;123(2): 834-873. [17] CORREA S, GROSSKOPF AK, LOPEZ HERNANDEZ H, et al. Translational Applications of Hydrogels. Chem Rev. 2021; 121(18):11385-11457. [18] 高晨珊,张亚莉,侯磊.可注射水凝胶治疗心肌梗死[J].国际心血管病杂志,2023, 50(1):9-12. [19] LEE LC, WALL ST, KLEPACH D, et al. Algisyl-LVR™ with coronary artery bypass grafting reduces left ventricular wall stress and improves function in the failing human heart. Int J Cardiol. 2013;168(3):2022-2028. [20] MELLATI A, HASANZADEH E, GHOLIPOURMALEKABADI M, et al. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: A review. Mater Sci Eng C Mater Biol Appl. 2021;131:112489. [21] 李树成,郭炳辰,高殿钰,等.可注射水凝胶在慢性心力衰竭治疗中的前景与挑战[J].中国介入心脏病学杂志,2024, 32(8):451-456. [22] LIAO J, HOU B, HUANG H. Preparation, properties and drug controlled release of chitin-based hydrogels: An updated review. Carbohydr Polym. 2022;283:119177. [23] MO C, LUO R, CHEN Y. Advances in the Stimuli-Responsive Injectable Hydrogel for Controlled Release of Drugs. Macromol Rapid Commun. 2022;43(10):e2200007. [24] ZHANG L, BEI Z, LI T, et al. An injectable conductive hydrogel with dual responsive release of rosmarinic acid improves cardiac function and promotes repair after myocardial infarction. Bioact Mater. 2023;29:132-150. [25] 郑月丹,王晓玲,冯俊峰,等.刺激响应型可注射水凝胶在药物控释领域的研究进展[J].分子材料科学与工程,2024, 40(9):164-172. [26] 吕永波,王娟.干细胞在治疗急性心肌梗死中应用的研究进展[J].中国临床新医学,2024,17(9):1061-1065. [27] ZHAO L, NIU L, LIANG H, et al. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl Mater Interfaces. 2017; 9(43):37563-37574. [28] VEGAD U, PATEL M, KHUNT D, et al. pH stimuli-responsive hydrogels from non-cellulosic biopolymers for drug delivery. Front Bioeng Biotechnol. 2023; 11:1270364. [29] DAI S, RAVI P, TAM KC. pH-Responsive polymers: synthesis, properties and applications. Soft Matter. 2008;4(3): 435-449. [30] KWON SS, KONG BJ, PARK SN. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose-hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur J Pharm Biopharm. 2015;92: 146-154. [31] HU C, LIU W, LONG L, et al. Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomaterials. 2022;290:121849. [32] NOUREEN S, NOREEN S, GHUMMAN SA, et al. A novel pH-responsive hydrogel system based on Prunus armeniaca gum and acrylic acid: Preparation and evaluation as a potential candidate for controlled drug delivery. Eur J Pharm Sci. 2023;189:106555. [33] KORNFELD OS, HWANG S, DISATNIK MH, et al. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res. 2015;116(11):1783-1799. [34] GAO F, XIONG Z. Reactive Oxygen Species Responsive Polymers for Drug Delivery Systems. Front Chem. 2021;9:649048. [35] REGATO-HERBELLA M, MORHENN I, MANTIONE D, et al. ROS-Responsive 4D Printable Acrylic Thioether-Based Hydrogels for Smart Drug Release. Chem Mater. 2024;36(3):1262-1272. [36] PU M, CAO H, ZHANG H, et al. ROS-responsive hydrogels: from design and additive manufacturing to biomedical applications. Mater Horiz. 2024;11(16): 3721-3746. [37] ZHANG J, SUN D, LIAO Y, et al. Time-Released Black Phosphorus Hydrogel Accelerates Myocardial Repairing through Antioxidant and Motivates Macrophage Polarization Properties. Biomater Res. 2024; 28:0029. [38] LIAO X, SONG X, LI J, et al. An injectable co-assembled hydrogel blocks reactive oxygen species and inflammation cycle resisting myocardial ischemia-reperfusion injury. Acta Biomater. 2022;149:82-95. [39] PERTICI V, PIN-BARRE C, RIVERA C, et al. Degradable and Injectable Hydrogel for Drug Delivery in Soft Tissues. Biomacromolecules. 2019;20(1):149-163. [40] SURYAVANSHI P, MAHAJAN S, BANERJEE SK, et al. Synthesis and characterization of a pH/temperature-dual responsive hydrogel with promising biocompatibility features for stimuli-responsive 5-FU delivery. J Mater Chem B. 2024;12(21):5098-5110. [41] CHOI JH, LEE JS, YANG DH, et al. Development of a Temperature-Responsive Hydrogel Incorporating PVA into NIPAAm for Controllable Drug Release in Skin Regeneration. ACS Omega. 2023;8(46):44076-44085. [42] ROH YH, MOON JY, HONG EJ, et al. Microfluidic fabrication of biocompatible poly(N-vinylcaprolactam)-based microcarriers for modulated thermo-responsive drug release. Colloids Surf B Biointerfaces. 2018;172:380-386. [43] LI P, HU J, WANG J, et al. The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives. Bioengineering (Basel). 2023;10(2):165. [44] CAO Y, FAN R, ZHU K, et al. Advances in Functionalized Hydrogels in the Treatment of Myocardial Infarction and Drug-Delivery Strategies. ACS Appl Mater Interfaces. 2024;16(37):48880-48894. [45] LIANG J, LV R, LI M, et al. Hydrogels for the Treatment of Myocardial Infarction: Design and Therapeutic Strategies. Macromol Biosci. 2024;24(2):e2300302. [46] LI X, ZHOU J, LIU Z, et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials. 2014; 35(22):5679-5688. [47] HUANG S, FRANGOGIANNIS NG. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol. 2018;175(9):1377-1400. [48] YU J, ZHANG RF, MAO YL. Cerebellar fastigial nucleus electrostimulation attenuates inflammation in a Post-Infarction rat model by activating cholinergic anti-inflammatory pathway. Neurosci Lett. 2022;788:136860. [49] CHEN M, WANG S, LI X, et al. Non-invasive Autonomic Neuromodulation Is Opening New Landscapes for Cardiovascular Diseases. Front Physiol. 2020;11:550578. [50] SOMANN JP, ALBORS GO, NEIHOUSER KV, et al. Chronic cuffing of cervical vagus nerve inhibits efferent fiber integrity in rat model. J Neural Eng. 2018;15(3):036018. [51] WANG M, YIN Y. Magnetically Responsive Nanostructures with Tunable Optical Properties. J Am Chem Soc. 2016;138(20): 6315-6323. [52] BAO S, LU Y, ZHANG J, et al. Rapid improvement of heart repair in rats after myocardial infarction by precise magnetic stimulation on the vagus nerve with an injectable magnetic hydrogel. Nanoscale. 2023;15(7):3532-3541. [53] Kono H, Otaka F, Ozaki M. Preparation and characterization of guar gum hydrogels as carrier materials for controlled protein drug delivery. Carbohydr Polym. 2014; 111:830-840. [54] THAMBI T, PHAN VH, LEE DS. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol Rapid Commun. 2016;37(23):1881-1896. [55] CHEN X, LIU Z. A pH-Responsive Hydrogel Based on a Tumor-Targeting Mesoporous Silica Nanocomposite for Sustained Cancer Labeling and Therapy. Macromol Rapid Commun. 2016;37(18):1533-1539. [56] CHEN W, WANG C, LIU W, et al. A Matrix-Metalloproteinase-Responsive Hydrogel System for Modulating the Immune Microenvironment in Myocardial Infarction. Adv Mater.2023;35(13):e2209041. [57] ACCIARETTI F, VESENTINI S, CIPOLLA L. Fabrication Strategies Towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights. Chem Asian J. 2022;17(22):e202200797. [58] ZHANG X, LYU Y, LIU Y, et al. Artificial apoptotic cells/VEGF-loaded injectable hydrogel united with immunomodification and revascularization functions to reduce cardiac remodeling after myocardial infarction. Nano Today. 2021;39:101227. [59] MORONI L, BOLAND T, BURDICK JA, et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018; 36(4):384-402. [60] JAFARI A, AJJI Z, MOUSAVI A, et al. Latest Advances in 3D Bioprinting of Cardiac Tissues. Adv Mater Technol. 2022;7(11): 2101636. [61] FATIMI A, OKORO OV, PODSTAWCZYK D, et al. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels. 2022;8(3):179. [62] TAMAY DG, DURSUN USAL T, ALAGOZ AS, et al. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front Bioeng Biotechnol. 2019;7:164. [63] KABIRIAN F, MELA P, HEYING R. 4D Printing Applications in the Development of Smart Cardiovascular Implants. Front Bioeng Biotechnol. 2022;10:873453. [64] 黄宇翔,李琪,叶武,等.生物4D打印技术在心血管组织工程中的研究及应用进展[J].生物工程学报,2023,39(10): 4046-4056. [65] FABER L, YAU A, CHEN Y. Translational biomaterials of four-dimensional bioprinting for tissue regeneration. Biofabrication. 2024;16(1):012001. [66] CHATTERJEE S, HUI PC. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers (Basel). 2021; 13(13):2086. [67] 张雅楠,许锋.心脏脱细胞基质水凝胶制备及其对心肌梗死修复的研究进展[J].心血管病学进展,2023,44(9):823-827. [68] SUN Y, ZHAO X, ZHANG Q, et al. An immunoregulatory and metabolism-improving injectable hydrogel for cardiac repair after myocardial infarction. Regen Biomater. 2025;12:rbae131. [69] ISHIKAWA K. The Transgenic Diabetic Pig Heart: A “Sweet Heart” for Translational Cardiovascular Research. J Am Coll Cardiol. 2017;69(2):144-146. |
| [1] | Liu Hongjie, Mu Qiuju, Shen Yuxue, Liang Fei, Zhu Lili. Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1929-1939. |
| [2] | Guo Yuchao, Ni Qianwei, Yin Chen, Jigeer·Saiyilihan, Gao Zhan . Quaternized chitosan hemostatic materials: synthesis, mechanism, and application [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2091-2100. |
| [3] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [4] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [5] | Wang Zilin, Mu Qiuju, Liu Hongjie, Shen Yuxue, Zhu Lili. Protective effects of platelet-rich plasma hydrogel on oxidative damage in L929 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 771-779. |
| [6] | Zhao Hongxia, Sun Zhengwei, Han Yang, Wu Xuechao , Han Jing. Osteogenic properties of platelet-rich fibrin combined with gelatin methacryloyl hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 809-817. |
| [7] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [8] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [9] |
Zhang Bo, Zhang Zhen, Jiang Dong.
Tannic acid modified interpenetrating network hydrogel promotes tissue remodeling of ruptured Achilles tendon after surgery#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 721-729.
|
| [10] | Li Yonghang, Li Wenming, Yan Caiping, Wang Xingkuan, Xiang Chao, Zhang Yuan, Jiang Ke, Chen Lu. Critical bone defect repaired with anti-fibrosis and “H”-type core-shell bionic scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3420-3431. |
| [11] | He Rui, Li Chongyi, Wang Ruiyao, Zeng Dan, Fan Daidi. Application of MXene-based hydrogels in wound repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3486-3493. |
| [12] | Liu Zhongyu, Li Wenya, Fan Yonghong, Lyu Shuang, Pei Juan, Chen Yaqin, Liu Beiyu, Sun Hongyu. Methacrylated dermal extracellular matrix hydrogel promotes repair of abdominal wall defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2074-2082. |
| [13] | Zhao Wenqi, Yu Haichi, Song Yiru, Yuan Tianyang, Liu Qinyi. Platelet-rich plasma and hydrogel for spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2189-2200. |
| [14] | Fang Yulu, Yi Bingcheng, Shen Yanbing, Tang Han, Zhang Yanzhong. Potential of corn husk fibers reinforced chitosan-based hydrogels in cartilage tissue engineering scaffold [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5493-5501. |
| [15] | Yu Xingge, Lin Kaili. Application of nanocomposite hydrogels in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5441-5446. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||