Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5756-5769.doi: 10.12307/2026.133
Previous Articles Next Articles
Effects of piRNA CFAPIR in doxorubicin-induced ferroptosis models of rat and human cardiomyocytes
Tao Xiangyu, Wang Shuang, Li Yuhan, Cao Jimin, Sun Teng
- Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Key Laboratory of Cellular Physiology of Shanxi Province, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2025-03-11Accepted:2025-08-08Online:2026-08-08Published:2025-12-27 -
Contact:Sun Teng, PhD, Associate professor, Doctoral supervisor, Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Key Laboratory of Cellular Physiology of Shanxi Province, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Tao Xiangyu, MS candidate, Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Key Laboratory of Cellular Physiology of Shanxi Province, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:National Natural Science Foundation of China (General Program), No. 82170294 (to ST); National Natural Science Foundation of China for the Youth, No. 81800268 (to ST); National Natural Science Foundation of China (General Program), No. 82170523 (to CJM); The Central Leading Local Science and Technology Development Fund Project, No. YDZJSX2022A061 (to ST); Shanxi Province Higher Education “Billion Project” Science and Technology Guidance Project, No. BYJL006 (to ST)
CLC Number:
Cite this article
Tao Xiangyu, Wang Shuang, Li Yuhan, Cao Jimin, Sun Teng. Effects of piRNA CFAPIR in doxorubicin-induced ferroptosis models of rat and human cardiomyocytes[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5756-5769.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
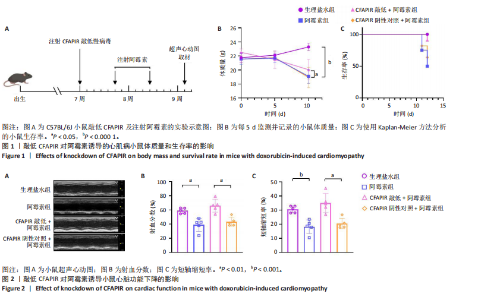
2.1 实验动物数量分析 实验选用雄性C57BL/6J小鼠68只,全部进入结果。 2.2 在阿霉素诱导的小鼠心脏中CFAPIR水平显著升高 阿霉素是常用的临床抗癌药物,在治疗过程中具有严重的心脏毒副作用,会造成心功能障碍和心肌损伤,从而诱发心肌病。为了探究piRNA CFAPIR是否参与阿霉素诱导心肌病的病理过程,构建了体内阿霉素诱导心肌病模型。 2.2.1 心肌病小鼠模型构建成功 小鼠腹腔注射累积剂量为16 mg/mL的阿霉素后,通过检测小鼠体质量变化、生存率,以及小鼠心功能和心肌损伤指标评估造模效果。结果显示,与对照组相比,阿霉素处理组小鼠生存率降低;体质量显著减轻(P < 0.000 1);心脏射血分数(P < 0.01)和短轴缩短率(P < 0.001)显著降低;心脏质量/胫骨长比值显著降低(P < 0.000 1);心肌细胞横截面积显著减小(P < 0.000 1);血清中乳酸脱氢酶活性显著增强(P < 0.001);心脏纤维化程度显著加重(P < 0.001),见图1-5。以上结果表明,阿霉素诱导心肌病小鼠模型构建成功。 2.2.2 阿霉素诱导心肌病模型中CFAPIR的水平 分离小鼠心脏组织并提取其中总RNA,通过qRT-PCR实验检测CFAPIR的水平。结果显示,与生理盐水组相比,阿霉素组小鼠心脏组织中CFAPIR的水平显著升高14倍左右(P < 0.000 1),见图6。该结果表明CFAPIR可能参与调控阿霉素诱导的心肌病。 2.3 敲低CFAPIR抑制阿霉素诱导的小鼠体质量减轻和生存率降低 为了探究CFAPIR在阿霉素诱导心肌病中的作用,实验构建了CFAPIR敲低慢病毒,心脏原位注射CFAPIR敲低慢病毒或其阴性对照慢病毒后,每5 d腹腔注射生理盐水或阿霉素1次,持续2次,同时,每5 d监测并记录一次小鼠的体质量,以及每天观察小鼠的存活情况至实验结束,见图1A。结果显示,与生理盐水组相比,阿霉素组小鼠的体质量明显减轻(P < 0.000 1),生存率显著降低。然而,与CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低后显著增加了小鼠体质量(P < 0.05)和小鼠的生存率,见图1B,C。以上结果表明,敲低CFAPIR明显改善阿霉素诱导的小鼠体质量减轻和生存率降低。 2.4 敲低CFAPIR改善阿霉素诱导的心功能障碍 为了探究CFAPIR在阿霉素诱导的小鼠心功能中的作用,通过超声心动图评价小鼠的心脏功能。结果显示,与生理盐水组相比,阿霉素组小鼠的射血分数(P < 0.01)和短轴缩短率(P < 0.001)显著降低。然而,与"
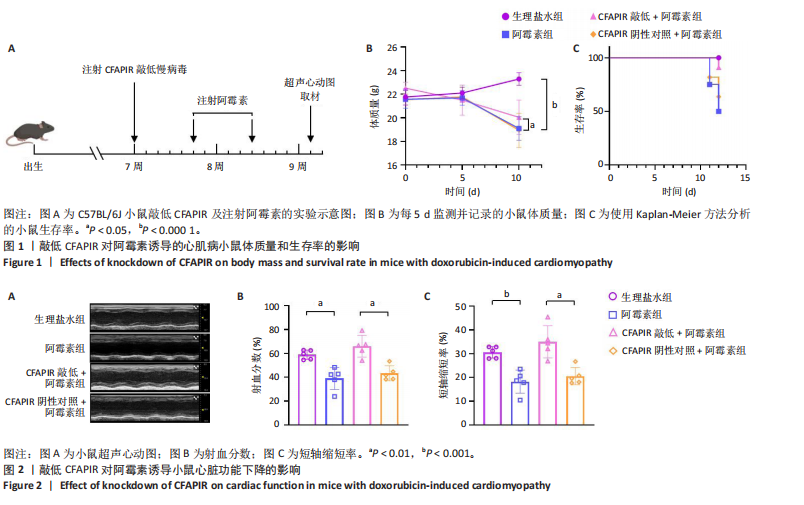
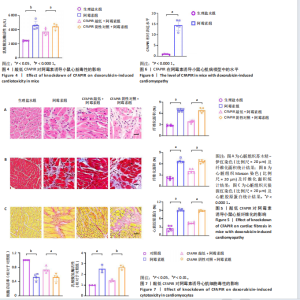
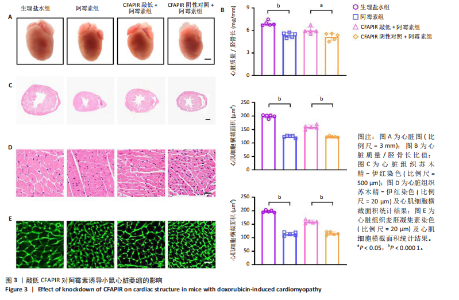
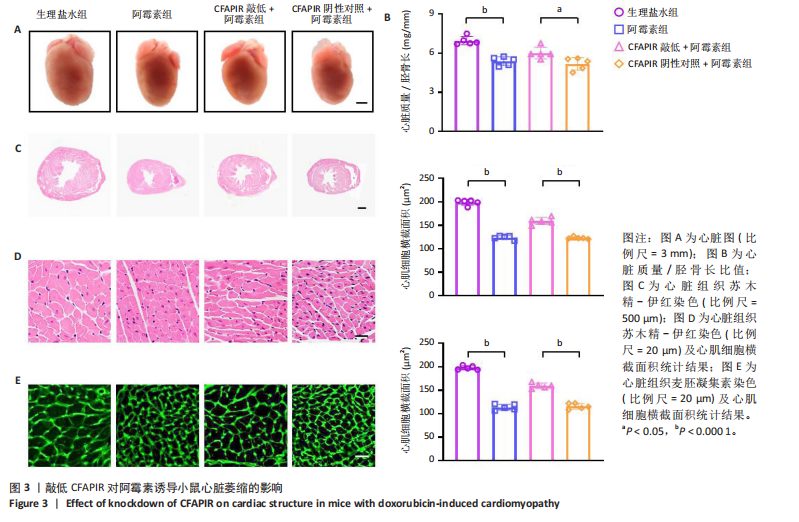
CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低+阿霉素组小鼠的射血分数和短轴缩短率显著升高(P < 0.01),见图2。以上结果表明,敲低CFAPIR显著改善了阿霉素诱导的心功能障碍。 2.5 敲低CFAPIR减弱阿霉素诱导的小鼠心脏萎缩 为了探究CFAPIR在阿霉素诱导的小鼠心脏结构改变中的作用,测量记录小鼠心脏体积、质量和胫骨长度。结果显示,与生理盐水组相比,阿霉素组小鼠心脏体积明显减小,心脏质量/胫骨长比值显著降低(P < 0.000 1),而这一现象被CFAPIR敲低显著减弱,见图3A,B。进一步通过心脏组织病理学染色进行验证,苏木精-伊红染色显示小鼠心脏横截面积显著减小,见图3C,苏木精-伊红染色(P < 0.000 1)和麦胚凝集素染色(P < 0.000 1)结果均显示心肌细胞横截面积显著减小,见图3D,E。然而,与CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低后显著抑制了阿霉素诱导的心脏横截面积和心肌细胞横截面积(P < 0.000 1)的减小,见图3C-E。以上结果表明,敲低CFAPIR显著改善了阿霉素诱导的小鼠心脏萎缩和心肌细胞缩小。 2.6 敲低CFAPIR抑制阿霉素诱导的小鼠心脏毒性 为了探究CFAPIR在阿霉素诱导心脏毒性中的作用,收集小鼠血清进行乳酸脱氢酶活性检测。结果显示,与生理盐水组相比,阿霉素组小鼠中乳酸脱氢酶活性显著增强(P < 0.000 1),然而,与阴性对照+阿霉素组相比,CFAPIR敲低后显著抑制了阿霉素诱导的乳酸脱氢酶活性的增强(P < 0.05),见图4。该结果表明,敲低CFAPIR显著减弱了阿霉素诱导的小鼠心脏毒性。 2.7 敲低CFAPIR减弱阿霉素诱导的小鼠心脏纤维化 为了探究CFAPIR在阿霉素诱导小鼠心肌损伤中的作用,通过苏木精-伊红染色、Masson染色检测小鼠心脏组织的纤维化程度,通过天狼猩红染色检测小鼠心脏组织胶原蛋白含量。结果显示,与生理盐水组相比,阿霉素组小鼠的心脏纤维化面积显著增大(P < 0.000 1),胶原蛋白含量显著增多(P < 0.000 1)。然而,与CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低后显著抑制了阿霉素诱导的心脏纤维化程度加重,显著降低了心脏纤维化面积(P < 0.000 1)和胶原蛋白含量(P < 0.000 1),见图5A-C。以上结果表明,敲低CFAPIR显著减弱了阿霉素诱导的心脏纤维化。 2.8 在阿霉素诱导的心肌细胞中CFAPIR水平显著升高 铁死亡是阿霉素诱导心肌病的重要机制,为了探究CFAPIR调控阿霉素诱导心肌病是否靶向心肌细胞铁死亡,使用3 μmol/L阿霉素处理AC16人心肌细胞24 h,成功构建了阿霉素诱导心肌细胞铁死亡模型,见图7,8。随后检测CFAPIR的水平,结果显示,与对照组相比,阿霉素组细胞中CFAPIR水平显著上调平均50倍左右(P < 0.01),见图9。以上结果提示CFAPIR可能参与了阿霉素诱导心肌细胞铁死亡的调控。 2.9 敲低CFAPIR减轻阿霉素诱导的心肌细胞损伤 为了探究CFAPIR在阿霉素诱导心肌细胞损伤和心肌"
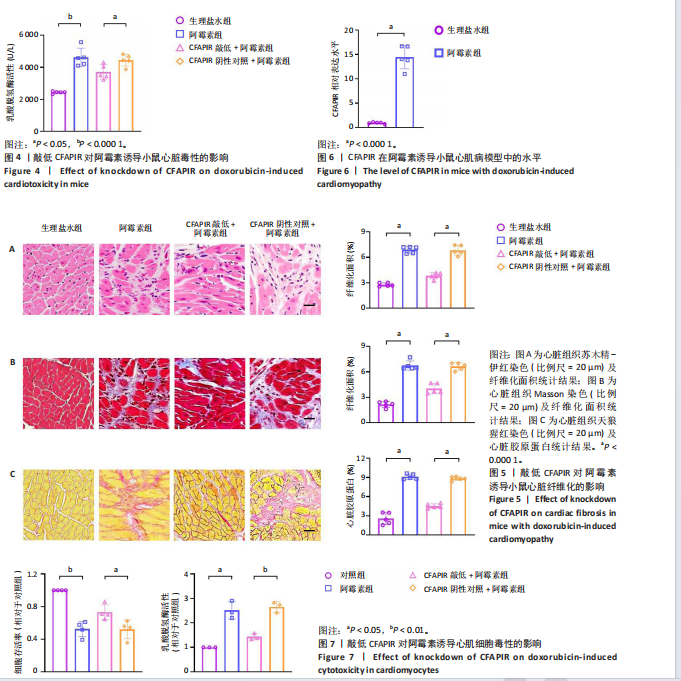
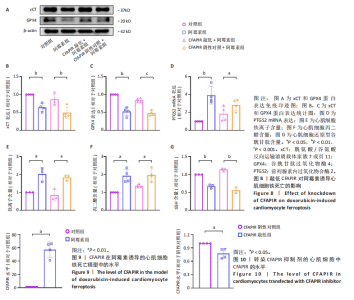
细胞铁死亡中的作用,合成了CFAPIR特异性抑制剂和其阴性对照,分别转染至AC16心肌细胞,结果显示CFAPIR特异性抑制剂显著降低了CFAPIR的表达水平(P < 0.05),见图10。进一步探究CFAPIR在阿霉素诱导心肌细胞损伤中的作用。CCK-8和乳酸脱氢酶活性实验结果显示,阿霉素显著降低心肌细胞的存活率(P < 0.01),增加细胞中乳酸脱氢酶活性(P < 0.05)。然而,与CFAPIR阴性对照+阿霉素组相比,敲低CFAPIR显著抑制阿霉素诱导的细胞存活率降低(P < 0.05),以及乳酸脱氢酶活性增强(P < 0.01),见图7。以上结果表明,抑制CFAPIR减轻了阿霉素诱导的心肌细胞毒性。 2.10 敲低CFAPIR抑制阿霉素诱导的心肌细胞铁死亡 实验检测了CFAPIR在阿霉素诱导心肌细胞铁死亡中的作用。结果显示,与对照组相比,阿霉素处理后的心肌细胞中铁死亡标志物xCT和GPX4的表达水平显著下降(P < 0.01),前列腺素内过氧化物合酶2的mRNA水平显著升高(P < 0.01);总铁离子含量显著增多(P < 0.05);丙二醛含量显著增多(P < 0.05);还原型谷胱甘肽含量显著减少(P < 0.01)。然而,与CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低后显著上调细胞中xCT (P < 0.01)和GPX4 (P < 0.001)的表达水平;显著下调前列腺素内过氧化物合酶2的mRNA水平(P < 0.05);显著减少细胞中铁离子含量(P < 0.05);显著减少丙二醛含量(P < 0.05);显著增加细胞中还原型谷胱甘肽含量(P < 0.01),见图8。以上结果表明,敲低CFAPIR显著抑制阿霉素诱导的心肌细胞铁死亡。"
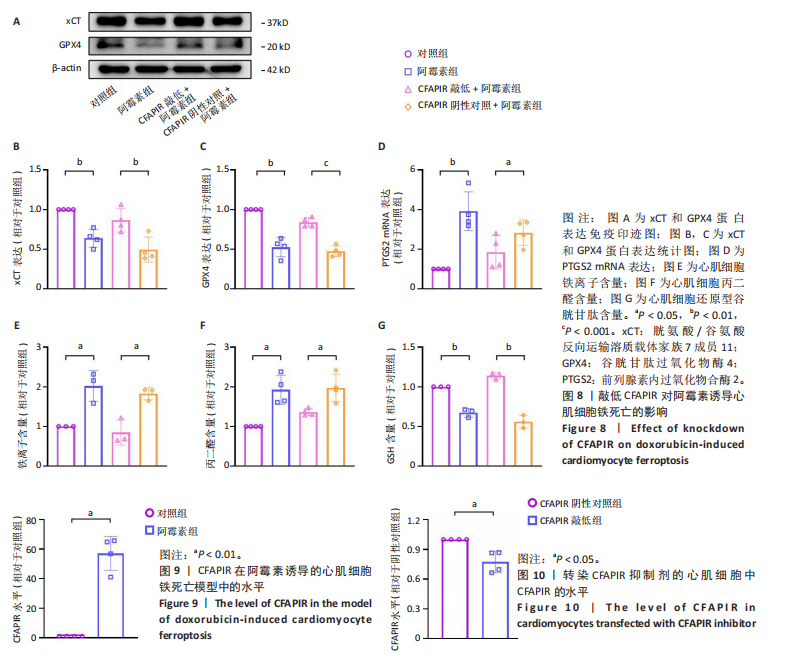
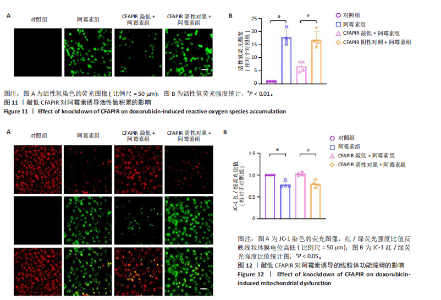
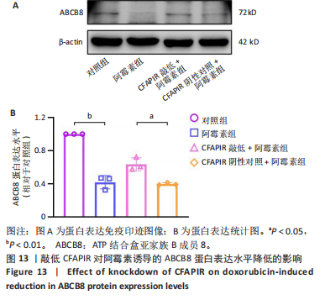
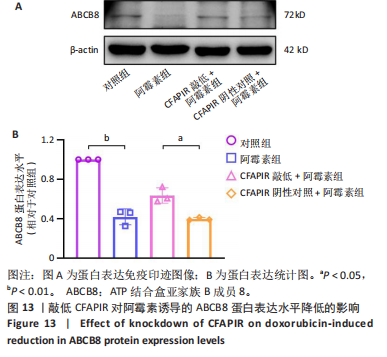
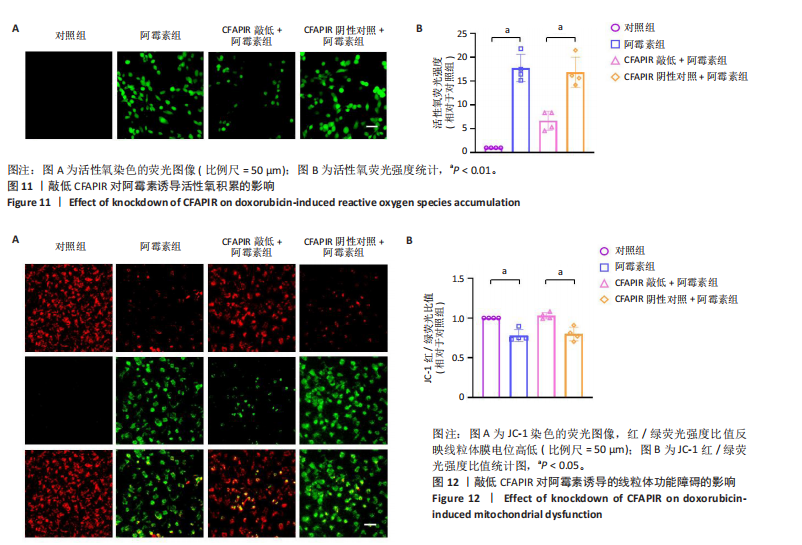
2.11 敲低CFAPIR减弱阿霉素诱导的心肌细胞活性氧积累 由于氧化应激是铁死亡过程中发生脂质过氧化的重要因素,活性氧的大量生成促进铁死亡发生。因此,探究了CFAPIR在阿霉素诱导的心肌细胞活性氧积累中的作用。使用DCFH-DA荧光探针检测活性氧水平。结果显示,与对照组相比,阿霉素显著增加了心肌细胞中活性氧水平(P < 0.01),而这一现象被CFAPIR的敲低显著减弱(P < 0.01),见图11。 2.12 敲低CFAPIR抑制阿霉素诱导的线粒体功能障碍 由于线粒体是铁代谢和活性氧生成的主要场所,二者都是参与铁死亡的关键因素,于是探究了CFAPIR在阿霉素诱导的心肌细胞线粒体功能障碍中的作用。通过JC-1荧光探针检测线粒体膜电位水平。正常细胞的线粒体膜电位较高,JC-1聚集在线粒体基质中,形成聚合物,可以产生红色荧光;当细胞受损线粒体膜电位较低时,JC-1则为单体,产生绿色荧光,因此,JC-1的红/绿荧光强度比值反映线粒体膜电位变化[44]。结果显示,与对照组相比,阿霉素显著减弱了JC-1的红/绿荧光强度比值(P < 0.05),即线粒体膜电位减小。然而,与CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低+阿霉素组中JC-1的红/绿荧光强度比值显著升高(P < 0.05),即显著增大了阿霉素降低的线粒体膜电位,见图12。以上结果表明,敲低CFAPIR显著改善了阿霉素诱导的线粒体功能障碍。 2.13 CFAPIR调控铁转运蛋白ABCB8 为了进一步探索CFAPIR调控阿霉素诱导心肌细胞铁死亡和线粒体功能障碍的分子机制,鉴定了CFAPIR的下游靶标。ABCB8是一种线粒体铁转运蛋白,调控线粒体铁外流,有研究报道阿霉素诱导的心脏毒性和心脏铁超载均与ABCB8的耗竭密切相关。在阿霉素诱导心肌细胞铁死亡模型中,CFAPIR是否与ABCB8相互作用尚不清楚,故开展了相关研究。实验结果显示,与对照组相比,阿霉素处理的心肌细胞中ABCB8表达水平显著降低(P < 0.01),而与CFAPIR阴性对照+阿霉素组相比,CFAPIR敲低显著上调了ABCB8的表达水平(P < 0.05),见图13。以上结果表明,在阿霉素诱导心肌细胞铁死亡模型中,CFAPIR调控了ABCB8的表达,且可能通过靶向ABCB8发挥调控阿霉素诱导心肌细胞铁死亡和心肌病的作用。"
| [1] CHEN Y, SHI S, DAI Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed Pharmacother. 2022; 156:113903. [2] WILLIAMS PA, ZAIDI SK, SENGUPTA R. AACR Cancer Progress Report 2023: Advancing the Frontiers of Cancer Science and Medicine. Clin Cancer Res. 2023;29(19):3850-3851. [3] LI D, YANG Y, WANG S, et al. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 2021;46:102089. [4] CHRISTIDI E, BRUNHAM LR. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12(4):339. [5] WU L, ZHANG Y, WANG G, et al. Molecular Mechanisms and Therapeutic Targeting of Ferroptosis in Doxorubicin-Induced Cardiotoxicity. JACC Basic Transl Sci. 2024;9(6):811-826. [6] VITALE R, MARZOCCO S, POPOLO A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int J Mol Sci. Int J Mol Sci. 2024;25(13):7477. [7] FABIANI I, AIMO A, GRIGORATOS C, et al. Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail Rev. 2021;26(4):881-890. [8] SCHIRONE L, D’AMBROSIO L, FORTE M, et al.Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay. Cells. 2022;11(13):2000. [9] BELGER C, ABRAHAMS C, IMAMDIN A, et al. Doxorubicin-induced cardiotoxicity and risk factors. Int J Cardiol Heart Vasc. 2024;50:101332. [10] FANG X, ARDEHALI H, MIN J, et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7-23. [11] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death]. Cell. 2012;149(5):1060-1072. [12] WU YT, ZHANG GY, LI L, et al. Salvia miltiorrhiza suppresses cardiomyocyte ferroptosis after myocardial infarction by activating Nrf2 signaling. J Ethnopharmacol. 2024;330:118214. [13] QU Z, PANG X, MEI Z, et al. The positive feedback loop of the NAT10/Mybbp1a/p53 axis promotes cardiomyocyte ferroptosis to exacerbate cardiac I/R injury. Redox Biol. 2024;72:103145. [14] CUI J, CHEN Y, YANG Q, et al. Protosappanin A Protects DOX-Induced Myocardial Injury and Cardiac Dysfunction by Targeting ACSL4/FTH1 Axis-Dependent Ferroptosis. Adv Sci (Weinh). 2024;11(34): e2310227. [15] LIU P, ZHANG Z, CAI Y, et al. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94: 102201. [16] ZHANG W, QIAN S, TANG B, et al. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J Cell Mol Med. 2023;27(20):3075-3089. [17] GONG Y, YANG H, CHEN T, et al. USP38 exacerbates myocardial injury and malignant ventricular arrhythmias after ischemia/reperfusion by promoting ferroptosis through the P53/SLC7A11 pathway. Int Immunopharmacol. 2025;145:113727. [18] XU X, XU XD, MA MQ, et al. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed Pharmacother. 2024;171:116112. [19] HE X, XIONG Y, LIU Y, et al. Ferrostatin-1 inhibits ferroptosis of vascular smooth muscle cells and alleviates abdominal aortic aneurysm formation through activating the SLC7A11/GPX4 axis. Faseb J. 2024; 38(2):e23401. [20] QIU H, HUANG S, LIU Y, et al. Idebenone alleviates doxorubicin-induced cardiotoxicity by stabilizing FSP1 to inhibit ferroptosis. Acta Pharm Sin B. 2024;14(6):2581-2597. [21] YANG Y, REN J, ZHANG J, et al. FTO ameliorates doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via P53-P21/Nrf2 activation in a HuR-dependent m6A manner. Redox Biol. 2024;70:103067. [22] LIU D, CHENG X, WU H, et al. CREG1 attenuates doxorubicin-induced cardiotoxicity by inhibiting the ferroptosis of cardiomyocytes. Redox Biol. 2024;75:103293. [23] WU L, DU Y, WANG L, et al. Inhibition of METTL3 ameliorates doxorubicin-induced cardiotoxicity through suppression of TFRC-mediated ferroptosis. Redox Biol. 2024;72:103157. [24] FANG X, WANG H, HAN D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7): 2672-2680. [25] WANG K, LI FH, ZHOU LY, et al. HNEAP Regulates Necroptosis of Cardiomyocytes by Suppressing the m(5) C Methylation of Atf7 mRNA. Adv Sci (Weinh). 2023;10(34):e2304329. [26] CHEN B, SHI B, ZHOU Z, et al. Targeting a cardiac abundant and fibroblasts-specific piRNA (CFRPi) to attenuate and reverse cardiac fibrosis in pressure-overloaded heart failure. Transl Res. 2024;267: 10-24. [27] ZHOU Y, FANG Y, DAI C, et al. PiRNA pathway in the cardiovascular system: a novel regulator of cardiac differentiation, repair and regeneration. J Mol Med (Berl). 2021;99(12):1681-1690. [28] WU Z, YU X, ZHANG S, et al. Novel roles of PIWI proteins and PIWI-interacting RNAs in human health and diseases. Cell Commun Signal. 2023;21(1):343. [29] ZENG Q, WAN H, ZHAO S, et al. Role of PIWI-interacting RNAs on cell survival: Proliferation, apoptosis, and cycle. IUBMB Life. 2020;72(9): 1870-1878. [30] GARCIA-BORJA E, SIEGL F, MATEU R, et al. Critical appraisal of the piRNA-PIWI axis in cancer and cancer stem cells. Biomark Res. 2024; 12(1):15. [31] DENG X, LIAO T, XIE J, et al. The burgeoning importance of PIWI-interacting RNAs in cancer progression. Sci China Life Sci. 2024;67(4): 653-662. [32] JIANG M, HONG X, GAO Y, et al. piRNA associates with immune diseases. Cell Commun Signal. 2024;22(1):347. [33] PIEROULI K, PAPAKONSTANTINOU E, PAPAGEORGIOU L, et al. Role of non‑coding RNAs as biomarkers and the application of omics technologies in Alzheimer’s disease (Review). Int J Mol Med. 2023; 51(1):5. [34] LI M, YANG Y, WANG Z, et al. Piwi-interacting RNAs (piRNAs) as potential biomarkers and therapeutic targets for cardiovascular diseases. Angiogenesis. 2021;24(1):19-34. [35] LV L, YUAN K, LI J, et al. PiRNA CFAPIR inhibits cardiac fibrosis by regulating the muscleblind-like protein MBNL2. Biochim Biophys Acta Mol Basis Dis. 2024;1870(8):167456. [36] GAO XQ, ZHANG YH, LIU F, et al. The piRNA CHAPIR regulates cardiac hypertrophy by controlling METTL3-dependent N(6)-methyladenosine methylation of Parp10 mRNA. Nat Cell Biol. 2020;22(11):1319-1331. [37] WANG K, ZHOU LY, LIU F, et al. PIWI-Interacting RNA HAAPIR Regulates Cardiomyocyte Death After Myocardial Infarction by Promoting NAT10-Mediated ac(4) C Acetylation of Tfec mRNA. Adv Sci (Weinh). 2022;9(8):e2106058. [38] CHI H, CHAI Y, MA L, et al. The mechanism by which piR-000699 targets SLC39A14 regulates ferroptosis in aging myocardial ischemia/reperfusion injury. Acta Biochim Biophys Sin (Shanghai). 2024;56(9):1352-1364. [39] JIAO A, LIU H, WANG H, et al. piR112710 attenuates diabetic cardiomyopathy through inhibiting Txnip/NLRP3-mediated pyroptosis in db/db mice. Cell Signal. 2024;122:111333. [40] LIU Y, QI H, ZONG J, et al. Oral Piwi-Interacting RNA Delivery Mediated by Green Tea-Derived Exosome-Like Nanovesicles for the Treatment of Aortic Dissection. Adv Healthc Mater. 2024;13(30):e2401466. [41] HU C, ZHANG X, WEI W, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 2019;9(4):690-701. [42] SANGOMLA S, SAIFI MA, KHURANA A, et al. Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. J Trace Elem Med Biol. 2018; 47:53-62. [43] ZHOU L, LI R, LIU C, et al.Foxo3a inhibits mitochondrial fission and protects against doxorubicin-induced cardiotoxicity by suppressing MIEF2.Free Radic Biol Med. 2017;104:360-370. [44] TAI P, CHEN X, JIA G, et al. WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J Transl Med. 2023;21(1):823. [45] MENON A V, KIM J.Iron Promotes Cardiac Doxorubicin Retention and Toxicity Through Downregulation of the Mitochondrial Exporter ABCB8. Front Pharmacol. 2022;13:817951. [46] KITAKATA H, ENDO J, IKURA H, et al. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis.Int J Mol Sci. 2022;23(3):1414. [47] HUANG C, GUO Y, LI T, et al.Pharmacological activation of GPX4 ameliorates doxorubicin-induced cardiomyopathy.Redox Biol. 2024; 70:103024. [48] KCIUK M, GIELECIŃSKA A, MUJWAR S, et al. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells. 2023;12(4):659. [49] TADOKORO T, IKEDA M, IDE T, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9):e132747. [50] WANG X, RAMAT A, SIMONELIG M, et al. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat Rev Mol Cell Biol. 2023; 24(2):123-141. [51] ICHIKAWA Y, GHANEFAR M, BAYEVA M, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124(2):617-630. [52] LIU Z, ZHAO X. piRNAs as emerging biomarkers and physiological regulatory molecules in cardiovascular disease. Biochem Biophys Res Commun. 2024;711:149906. |
| [1] | Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807. |
| [2] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [3] | Zou Rongji, Yu Fangfang, Wang Maolin, Jia Zhuopeng. Triptolide inhibits ferroptosis and improves cerebral ischemia-reperfusion injury in a rat model of cerebral artery occlusion/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 873-881. |
| [4] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [5] | Zhou Wen, Yang Hongwei. Molecular mechanism and natural drug screening for ferroptosis-targeted therapy in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6051-6061. |
| [6] | Zheng Peng, Jia Xiaoning, Tao Jingwei, Fan Xiao. Tetramethylpyrazine improves iron metabolism disorders in a rat model of spinal cord injury via the Keap-1/Nrf2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6134-6141. |
| [7] | Huang Yushan, Wang Rongrong, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 pretreatment inhibits ferroptosis in endothelial cells in a rat model of spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5716-5727. |
| [8] | Liao Xingzhuan, Li Guangdi, Wu Yabin, Liu Xingyu, Wan Jiajia. Molecular mechanisms underlying non-coding RNA regulation of ferroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4713-4725. |
| [9] | Yu Le, Nan Songhua, Shi Zijian, He Qiqi, Li Zhenjia, Cui Yinglin. Mechanisms underlying mitophagy, ferroptosis, cuproptosis, and disulfidptosis in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4446-4456. |
| [10] | Liu Annan, Li Jianhui, Gao Wei, Li Xue, Song Jing, Xing Liping, Li Honglin. Bibliometric analysis of ferroptosis and Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4278-4288. |
| [11] | Chen Xinlong, Meng Tao, Wang Yaomin, Zhang Kefan, Li Jian, Shi Hui, Zhang Chenchen. Ferroptosis inhibitors in the treatment of osteoarthritis: diversity and multitarget characteristics [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4166-4179. |
| [12] | Yao Shunhua, Huang Caiding, Zhang Mengyu, Zhang Kexin, Yin Changjiang, Yang Kunbao. Huidouba inhibits ferroptosis in high glucose-cultured HK-2 cells to attenuate cell fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2774-2783. |
| [13] |
Zhang Yueting, Li Jinglin, Fu Zhenyi, Yan Fei, Gao Yu, Liu Jiaxin.
Endoplasmic reticulum stress promotes ferroptosis and aggravates cerebral ischemia-reperfusion injury#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2806-2813.
|
| [14] | Qi Xiang, Cao Shan, Chen Jian, Zhang Yijia, Liu Keke, Xu Zifu, Liu Wang, Fu Xiaoxiao, Yin Xiaolei. Screening of genes related to mitochondrial dysfunction and ferroptosis in atherosclerosis and target prediction of regulatory traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2641-2652. |
| [15] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||