Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (11): 2774-2783.doi: 10.12307/2026.124
Previous Articles Next Articles
Huidouba inhibits ferroptosis in high glucose-cultured HK-2 cells to attenuate cell fibrosis
Yao Shunhua, Huang Caiding, Zhang Mengyu, Zhang Kexin, Yin Changjiang, Yang Kunbao
- Institute of Traditional Chinese Medicine, Chengde Medical University, Chengde 067000, Hebei Province, China
-
Received:2025-03-06Accepted:2025-07-04Online:2026-04-18Published:2025-09-05 -
Contact:Yang Kunbao, PhD, Professor, Institute of Traditional Chinese Medicine, Chengde Medical University, Chengde 067000, Hebei Province, China Co-corresponding author: Yin Changjiang, MS, Associate professor, Institute of Traditional Chinese Medicine, Chengde Medical University, Chengde 067000, Hebei Province, China -
About author:Yao Shunhua, MS candidate, Institute of Traditional Chinese Medicine, Chengde Medical University, Chengde 067000, Hebei Province, China -
Supported by:the National Natural Science Foundation of China (General Program), No. 82474675 (to YKB); National Natural Science Foundation of China for the Youth, No. 81804217 (to YKB); “333 Talent Project” of Hebei Provincial Department of Human Resources and Social Affairs, No. C20231059 (to YKB)
CLC Number:
Cite this article
Yao Shunhua, Huang Caiding, Zhang Mengyu, Zhang Kexin, Yin Changjiang, Yang Kunbao. Huidouba inhibits ferroptosis in high glucose-cultured HK-2 cells to attenuate cell fibrosis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2774-2783.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
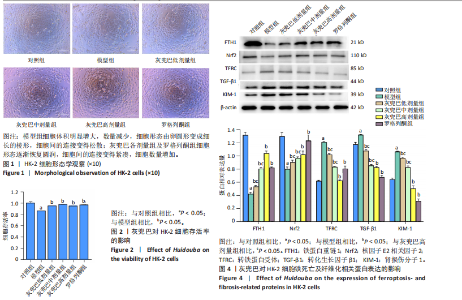
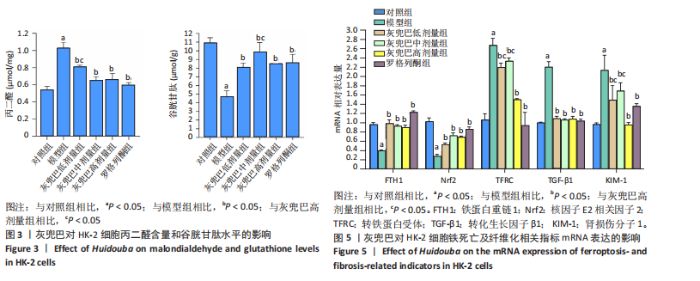
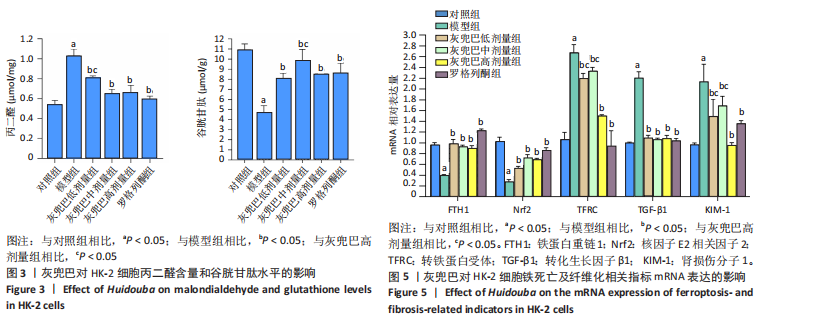
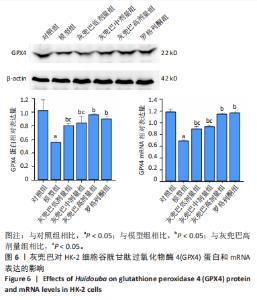
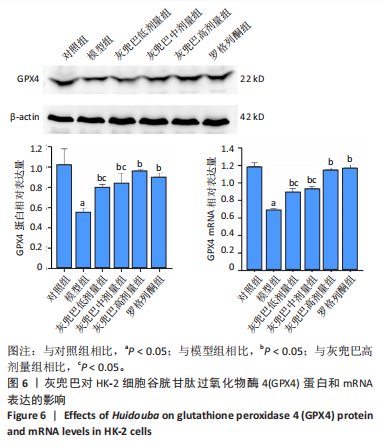
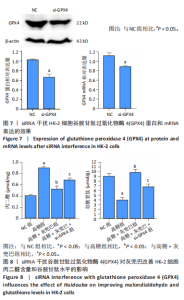
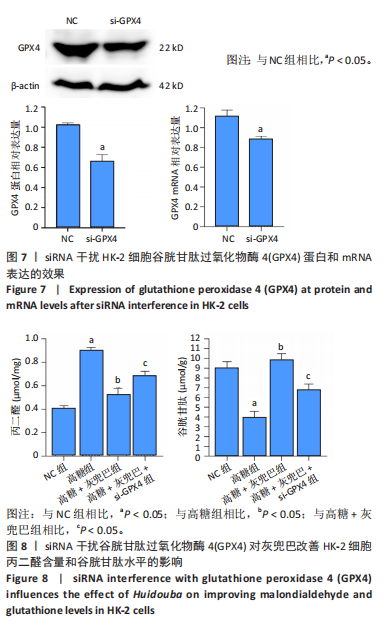
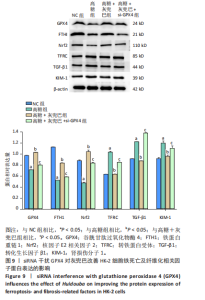
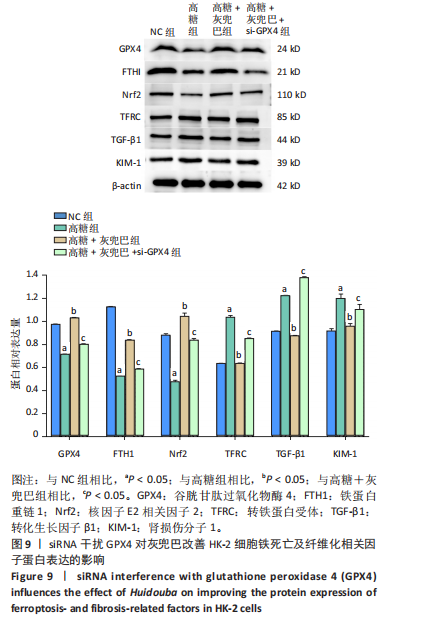
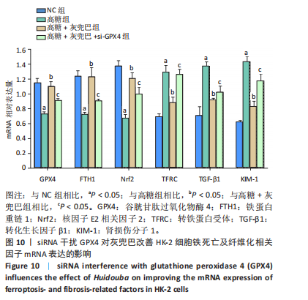
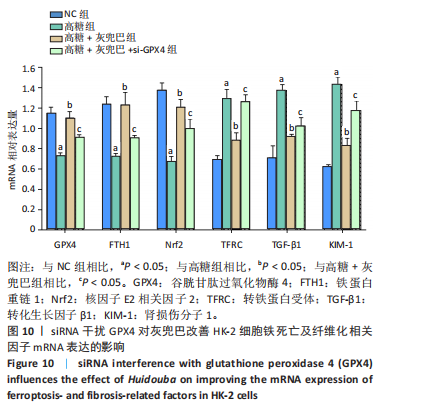
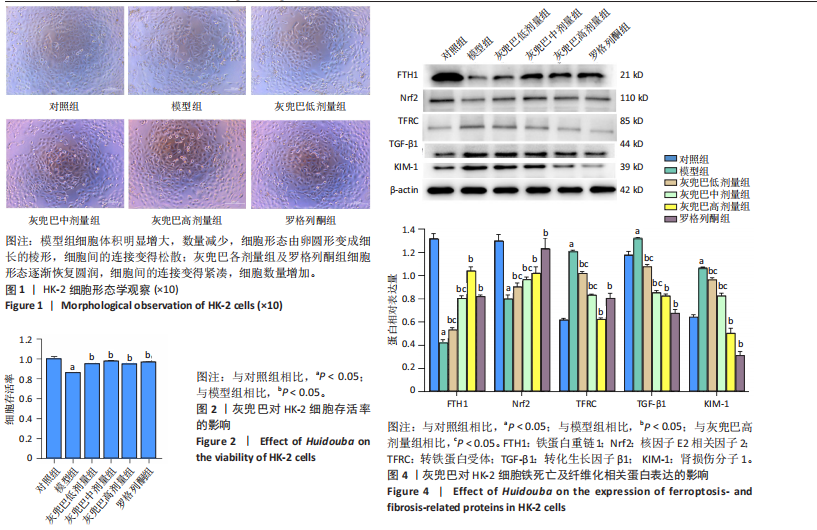
2.1 灰兜巴对高糖培养HK-2细胞铁死亡及纤维化的影响 2.1.1 HK-2细胞形态学观察 显微镜观察显示,对照组细胞呈鹅卵石状,细胞大小均一且连接紧密;模型组细胞体积明显增大,细胞数量减少,且细胞形态由卵圆形变成细长的棱形,细胞间的连接变得松散;灰兜巴各剂量组及罗格列酮组细胞形态逐渐恢复圆润,细胞间的连接变得紧凑,细胞数量增加;见图1。 2.1.2 灰兜巴对HK-2细胞存活率的影响 与对照组相比,模型组细胞存活率显著下降(P < 0.05);与模型组相比,灰兜巴各剂量组及罗格列酮组可不同程度提高HK-2细胞存活率(P < 0.05);灰兜巴各剂量组组间比较显示,细胞存活率差异无显著性意义(P > 0.05),见图2。 2.1.3 灰兜巴对HK-2细胞丙二醛含量和谷胱甘肽水平的影响 与对照组相比,模型组谷胱甘肽水平显著降低(P < 0.05),丙二醛含量显著升高(P < 0.05);与模型组相比,灰兜巴各剂量组及罗格列酮组可不同程度升高谷胱甘肽水平(P < 0.05),降低丙二醛含量(P < 0.05);灰兜巴各组间比较显示,灰兜巴低剂量组丙二醛含量高于灰兜巴高剂量组(P < 0.05),灰兜巴中剂量组谷胱甘肽水平高于灰兜巴高、低剂量组(P < 0.05),见图3。 2.1.4 灰兜巴对HK-2细胞铁死亡及纤维化相关蛋白表达水平的影响 与对照组相比,模型组铁蛋白重链1、核因子E2相关因子2蛋白表达水平显著降低(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达水平显著升高(P < 0.05);与模型组相比,灰兜巴各剂量组及罗格列酮组可不同程度升高铁蛋白重链1、核因子E2相关因子2蛋白表达水平(P < 0.05),降低转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达水平(P < 0.05);灰兜巴各组间比较显示,灰兜巴中、低剂量组铁蛋白重链1、核因子E2相关因子2蛋白表达水平低于灰兜巴高剂量组(P < 0.05),而转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达水平高于灰兜巴高剂量组(P < 0.05),见图4。 2.1.5 灰兜巴对HK-2细胞铁死亡及纤维化相关指标mRNA表达的影响 与对照组相比,模型组铁蛋白重链1、核因子E2相关因子2的mRNA表达显著降低(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1的mRNA表达显著升高(P < 0.05);与模型组相比,灰兜巴各剂量及罗格列酮组可不同程度升高铁蛋白重链1、核因子E2相关因子2的mRNA表达(P < 0.05),降低转铁蛋白受体、转化生长因子β1、肾损伤分子1的mRNA表达(P < 0.05),灰兜巴各组间比较显示,铁蛋白重链1、核因子E2相关因子2、转化生长因子β1的mRNA表达差异无显著性意义(P > 0.05),而灰兜巴中、低剂量组转铁蛋白受体、肾损伤分子1的mRNA表达均高于灰兜巴高剂量组(P < 0.05),见图5。 2.2 GPX4对灰兜巴抑制高糖培养HK-2细胞铁死亡减轻细胞纤维化作用效果的影响 2.2.1 灰兜巴对HK-2细胞GPX4蛋白和mRNA表达的影响 与对照组相比,模型组GPX4蛋白和mRNA表达显著降低(P < 0.05);与模型组相比,灰兜巴各剂量组及罗格列酮组可不同程度升高GPX4蛋白和mRNA表达(P < 0.05);灰兜巴各组间比较显示,灰兜巴中、低剂量组GPX4蛋白和mRNA表达均显著低于灰兜巴高剂量组(P < 0.05),见图6。 2.2.2 siRNA干扰HK-2细胞GPX4表达效果的观察 与NC组相比,si-GPX4组GPX4蛋白和mRNA表达显著降低(P < 0.05),见图7。 2.2.3 siRNA干扰GPX4对灰兜巴改善HK-2细胞丙二醛含量和谷胱甘肽水平作用效果的影响 与NC组相比,高糖组谷胱甘肽水平显著降低(P < 0.05),丙二醛含量显著升高(P < 0.05);与高糖相比,高糖+灰兜巴组谷胱甘肽水平显著升高(P < 0.05),丙二醛含量显著降低(P < 0.05);与高糖+灰兜巴组相比,高糖+灰兜巴+si-GPX4组谷胱甘肽水平显著降低(P < 0.05),丙二醛含量显著升高(P < 0.05),见图8。 2.2.4 siRNA干扰GPX4对灰兜巴改善HK-2细胞GPX4、铁蛋白重链1、核因子E2相关因子2、转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达的影响 与NC组相比,高糖组GPX4、铁蛋白重链1、核因子E2相关因子2蛋白表达显著降低(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达显著升高(P < 0.05);与高糖组相比,高糖+灰兜巴组GPX4、铁蛋白重链1、核因子E2相关因子2蛋白表达显著升高(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达显著降低(P < 0.05);与高糖+灰兜巴组相比,高糖+灰兜巴+si-GPX4组GPX4、铁蛋白重链1、核因子E2相关因子2蛋白表达显著降低(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1蛋白表达显著升高(P < 0.05),见图9。 2.2.5 siRNA干扰GPX4对灰兜巴改善HK-2细胞GPX4、铁蛋白重链1、核因子E2相关因子2、转铁蛋白受体、转化生长因子β1、肾损伤分子1的mRNA表达的影响 见图10。与NC组相比,高糖组GPX4、铁蛋白重链1、核因子E2相关因子2的mRNA表达显著降低(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1的mRNA表达显著升高(P < 0.05);与高糖组相比,高糖+灰兜巴组GPX4、铁蛋白重链1、核因子E2相关因子2的mRNA表达显著升高(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1的mRNA表达显著降低(P < 0.05);与高糖+灰兜巴组相比,高糖+灰兜巴+si-GPX4组GPX4、铁蛋白重链1、核因子E2相关因子2的mRNA表达显著降低(P < 0.05),转铁蛋白受体、转化生长因子β1、肾损伤分子1的mRNA表达显著升高(P < 0.05)。"
| [1] SAGOO MK, GNUDI L. Diabetic Nephropathy: An Overview. Methods Mol Biol. 2020;2067:3-7. [2] BAI S, XIONG X, TANG B, et al. hsa-miR-199b-3p Prevents the Epithelial-Mesenchymal Transition and Dysfunction of the Renal Tubule by Regulating E-cadherin through Targeting KDM6A in Diabetic Nephropathy. Oxid Med Cell Longev. 2021;2021:8814163. [3] KUSHWAHA K, KABRA U, DUBEY R, et al. Diabetic Nephropathy: Pathogenesis to Cure. Curr Drug Targets. 2022;23(15):1418-1429. [4] ROCHETTE L, DOGON G, RIGAL E, et al. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci. 2022;24(1):449. [5] 陶倩, 刘念, 陈静,等. 铁死亡与肿瘤免疫[J]. 中南大学学报(医学版),2024,49(8):1309-1315. [6] HENNING Y, BLIND US, LARAFA S, et al. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022; 13(7):662. [7] TANG D, CHEN X, KANG R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107-125. [8] HE J, LI Z, XIA P, et al. Ferroptosis and ferritinophagy in diabetes complications. Mol Metab. 2022;60:101470. [9] URSINI F, MAIORINO M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med. 2020;152:175-185. [10] QIAO Y, SUN C, KAN S, et al. SRS 16-86 promotes diabetic nephropathy recovery by regulating ferroptosis. Exp Physiol. 2024;109(7):1199-1210. [11] LIU Z, NAN P, GONG Y, et al. Endoplasmic reticulum stress-triggered ferroptosis via the XBP1-Hrd1-Nrf2 pathway induces EMT progression in diabetic nephropathy. Biomed Pharmacother. 2023;164:114897. [12] TAN H, CHEN J, LI Y, et al. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol Med. 2022;28(1):58. [13] WU Q, HUANG F. Targeting ferroptosis as a prospective therapeutic approach for diabetic nephropathy. Ann Med. 2024;56(1):2346543. [14] LI S, ZHENG L, ZHANG J, et al. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435-449. [15] ZHANG Y, QU Y, CAI R, et al. Atorvastatin ameliorates diabetic nephropathy through inhibiting oxidative stress and ferroptosis signaling. Eur J Pharmacol. 2024;976:176699. [16] XIONG D, HU W, HAN X, et al. Rhein Inhibited Ferroptosis and EMT to Attenuate Diabetic Nephropathy by Regulating the Rac1/NOX1/β-Catenin Axis. Front Biosci (Landmark Ed). 2023;28(5):100. [17] WANG Y, BI R, QUAN F, et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol. 2020;888:173574. [18] CHEN H, ZHANG Y, MIAO Y, et al. Vitamin D inhibits ferroptosis and mitigates the kidney injury of prediabetic mice by activating the Klotho/p53 signaling pathway. Apoptosis. 2024;29(9-10):1780-1792. [19] LV S, LI H, ZHANG T, et al. San-Huang-Yi-Shen capsule ameliorates diabetic nephropathy in mice through inhibiting ferroptosis. Biomed Pharmacother. 2023;165:115086. [20] JIN T, CHEN C. Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem Toxicol. 2022;163:112892. [21] ZHANG S, LI Y, LIU X, et al. Carnosine alleviates kidney tubular epithelial injury by targeting NRF2 mediated ferroptosis in diabetic nephropathy. Amino Acids. 2023;55(9):1141-1155. [22] LV S, FAN L, CHEN X, et al. Jian-Pi-Gu-Shen-Hua-Yu Decoction Alleviated Diabetic Nephropathy in Mice through Reducing Ferroptosis. J Diabetes Res. 2024;2024:9990304. [23] FENG X, WANG S, SUN Z, et al. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front Endocrinol (Lausanne). 2021;12:626390. [24] FANG X, SONG J, CHEN Y, et al. LncRNA SNHG1 knockdown inhibits hyperglycemia induced ferroptosis via miR-16-5p/ACSL4 axis to alleviate diabetic nephropathy.J Diabetes Investig. 2023;14(9):1056-1069. [25] XUE M, TIAN Y, ZHANG H, et al. Curcumin nanocrystals ameliorate ferroptosis of diabetic nephropathy through glutathione peroxidase 4. Front Pharmacol. 2024;15:1508312. [26] ZHOU Y, HU T, ZENG H, et al. Naringenin Inhibits Ferroptosis in Renal Tubular Epithelial Cells of Diabetic Nephropathy Through SIRT1/FOXO3a Signaling Pathway. Drug Dev Res. 2025;86(1):e70044. [27] ZHANG S, ZHANG S, WANG H, et al. Vitexin ameliorated diabetic nephropathy via suppressing GPX4-mediated ferroptosis. Eur J Pharmacol. 2023;951:175787. [28] WU K, FEI L, WANG X, et al. ZIP14 is involved in iron deposition and triggers ferroptosis in diabetic nephropathy. Metallomics. 2022; 14(7):mfac034. [29] 杨坤宝, 庞宗然. 藏药灰兜巴治疗糖尿病的研究进展[J]. 中草药, 2017,48(8):1682-1686. [30] BAI YH, SHI DX, LU HY, et al.Hypoglycemic effects of Tibetan medicine Huidouba in STZ-induced diabetic mice and db/db mice. Chin Herb Med. 2021;13(2):202-209. [31] YANG K, BAI Y, YU N, et al. Huidouba Improved Podocyte Injury by Down-Regulating Nox4 Expression in Rats With Diabetic Nephropathy. Front Pharmacol. 2020;11:587995. [32] 张圣英. 灰兜巴通过激活PPARγ介导TGF-β/Smad信号通路抑制高糖诱导的HK-2细胞纤维化机制研究[D].承德:承德医学院,2023. [33] LU Q, CHEN Y B, YANG H, et al. Inactivation of TSC1 promotes epithelial-mesenchymal transition of renal tubular epithelial cells in mouse diabetic nephropathy.Acta Pharmacol Sin. 2019;40(12):1555-1567. [34] 刘东齐, 闫仕祺, 刘浩龙,等.芍药苷对糖尿病周围神经病变状态下线粒体输入途径蛋白TOM20的作用[J].中草药,2024,55(9): 2987-2995. [35] 刁俊玲, 伍敏, 黄荣凤,等. 新型PPARγ激动剂CMHX008对高糖诱导人肾小管上皮HK-2细胞纤维化的影响及机制[J].中国药理学通报,2019,35(10):1363-1369. [36] 谷李影, 杨胜富, 张启明,等. 香青兰总黄酮(TFDM)对高糖诱导的视网膜神经节细胞氧化损伤的影响及其机制[J]. 眼科新进展, 2024,44(12):937-942. [37] 罗羽, 王仙园, 杨云青.糖尿病肾病肾纤维化病变的发病机制研究进展[J].护理研究,2013,27(4):292-295. [38] LI J, SHU L, JIANG Q, et al. Oridonin ameliorates renal fibrosis in diabetic nephropathy by inhibiting the Wnt/β-catenin signaling pathway. Ren Fail. 2024;46(1):2347462. [39] WANG Y, YUE S, CAI F, et al. Treatment of berberine alleviates diabetic nephropathy by reducing iron overload and inhibiting oxidative stress. Histol Histopathol. 2023;38(9):1009-1016. [40] CHEN J, ZHANG MH, ZHANG L, et al. [Protective effects and mechanism of glycosides/phenol component of Moutan Cortex on renal injury of diabetic nephropathy rats]. Zhongguo Zhong Yao Za Zhi. 2016; 41(11):1990-1998. [41] MAO ZM, SHEN SM, WAN YG, et al. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J Ethnopharmacol. 2015;173:256-265. [42] WU X, LI H, WAN Z, et al. The combination of ursolic acid and empagliflozin relieves diabetic nephropathy by reducing inflammation, oxidative stress and renal fibrosis. Biomed Pharmacother. 2021; 144:112267. [43] DU N, XU Z, GAO M, et al. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des Devel Ther. 2018;12:3517-3524. [44] ARZUK E, ARMAğAN G. Genistein and daidzein induce ferroptosis in MDA-MB-231 cells. J Pharm Pharmacol. 2024;8:rgae106. [45] DODSON M, CASTRO-PORTUGUEZ R, ZHANG DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019; 23:101107. [46] NIELSEN SE, SCHJOEDT KJ, ASTRUP AS, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27(10):1144-1150. [47] MORI Y, AJAY AK, CHANG JH, et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 2021;33(5):1042-1061. [48] WANG H, YU X, LIU D, et al. VDR Activation Attenuates Renal Tubular Epithelial Cell Ferroptosis by Regulating Nrf2/HO-1 Signaling Pathway in Diabetic Nephropathy.Adv Sci (Weinh). 2024;11(10):e2305563. [49] JIA J, TAN R, XU L, et al. Hederagenin improves renal fibrosis in diabetic nephropathy by regulating Smad3/NOX4/SLC7A11 signaling-mediated tubular cell ferroptosis.Int Immunopharmacol. 2024;135:112303. [50] KIM S, KANG S W, JOO J, et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12(2):160. [51] SHAN XM, CHEN CW, ZOU DW, et al. Suppression of ferroptosis through the SLC7A11/glutathione/glutathione peroxidase 4 axis contributes to the therapeutic action of the Tangshenning formula on diabetic renal tubular injury. Chin Med. 2024;19(1):151. [52] HUANG J, CHEN G, WANG J, et al. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4). Bioengineered. 2022;13(3):6627-6637. [53] CHEN J, OU Z, GAO T, et al. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed Pharmacother. 2022;156:113953. [54] 孟凡欣, 王立英, 宋静静,等.灰兜巴多糖对糖尿病降糖作用的研究[J].时珍国医国药,2012,23(6):1557-1558. [55] 伍艳, 王小楠, 陈烽烽,等.灰兜巴活性成分治疗Ⅱ型糖尿病效果研究[J].中药材,2013,36(8):1313-1316. |
| [1] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [2] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [3] | Liu Kexin, , Hao Kaimin, Zhuang Wenyue, , Li Zhengyi. Autophagy-related gene expression in pulmonary fibrosis models: bioinformatic analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1129-1138. |
| [4] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [5] | Zou Rongji, Yu Fangfang, Wang Maolin, Jia Zhuopeng. Triptolide inhibits ferroptosis and improves cerebral ischemia-reperfusion injury in a rat model of cerebral artery occlusion/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 873-881. |
| [6] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [7] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [8] | Hong Runyang, Zhou Qiyue, Fan Zhencheng, Shi Yujie, Chen Hao, Pan Chun. Impact and mechanism of low-dose hexafluoropropylene oxide dimer acid exposure during pregnancy on renal toxicity in offspring mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2752-2763. |
| [9] |
Zhang Yueting, Li Jinglin, Fu Zhenyi, Yan Fei, Gao Yu, Liu Jiaxin.
Endoplasmic reticulum stress promotes ferroptosis and aggravates cerebral ischemia-reperfusion injury#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2806-2813.
|
| [10] | Jiao Taiqiang, Han Xingji, Li Xiangyang, Nan Yi, Yuan Ling, Li Jiaqing, Niu Yang. Mechanism by which Maxing Kugan Decoction intervenes in oleic acid-induced acute lung injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2430-2439. |
| [11] | Qi Xiang, Cao Shan, Chen Jian, Zhang Yijia, Liu Keke, Xu Zifu, Liu Wang, Fu Xiaoxiao, Yin Xiaolei. Screening of genes related to mitochondrial dysfunction and ferroptosis in atherosclerosis and target prediction of regulatory traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2641-2652. |
| [12] | Zhang Tingting, Li Yalong, Yue Haodi, Li Yanjun, Geng Xiwen, Zhang Yuwei, Liu Xiaozhuan. Protection of exosomes derived from bone marrow mesenchymal stem cells of different mouse ages on radiation-induced lung injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 1-9. |
| [13] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [14] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [15] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||