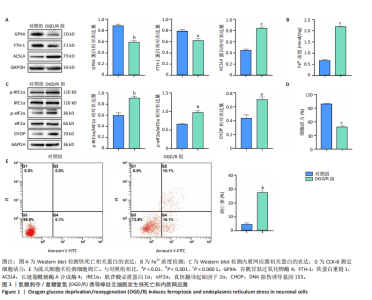
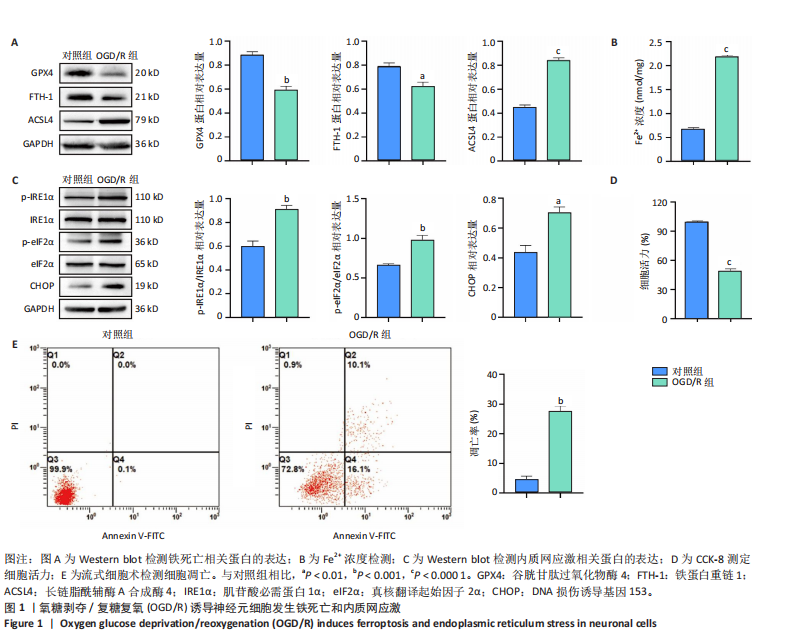
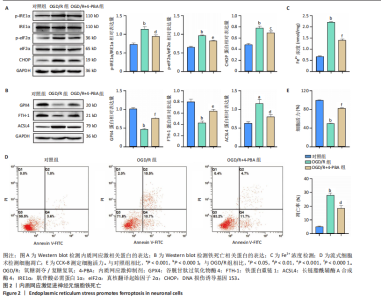
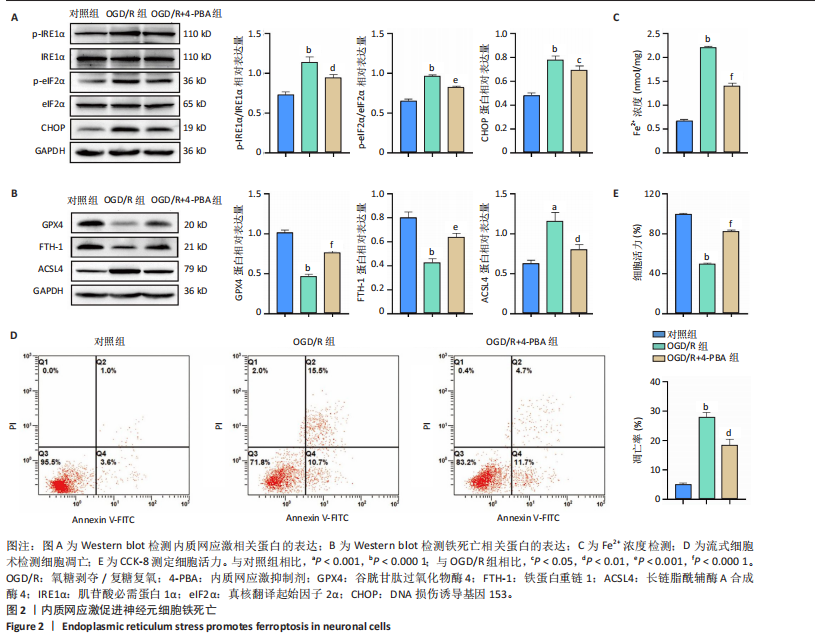
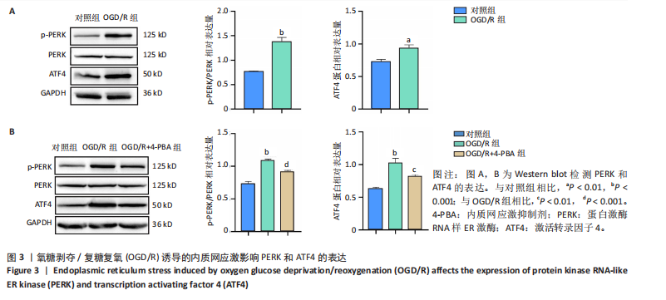
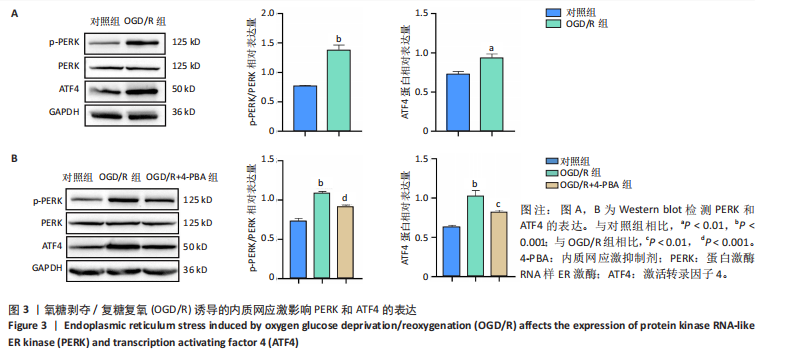
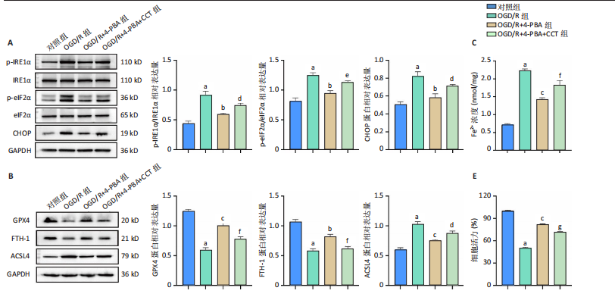
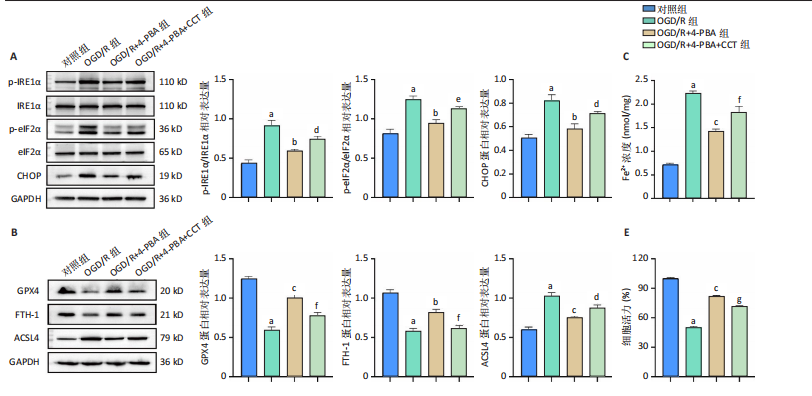
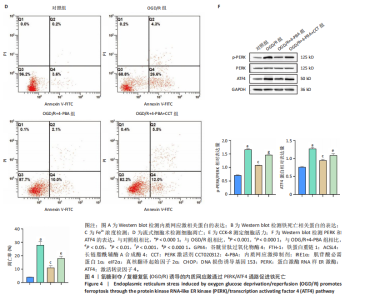
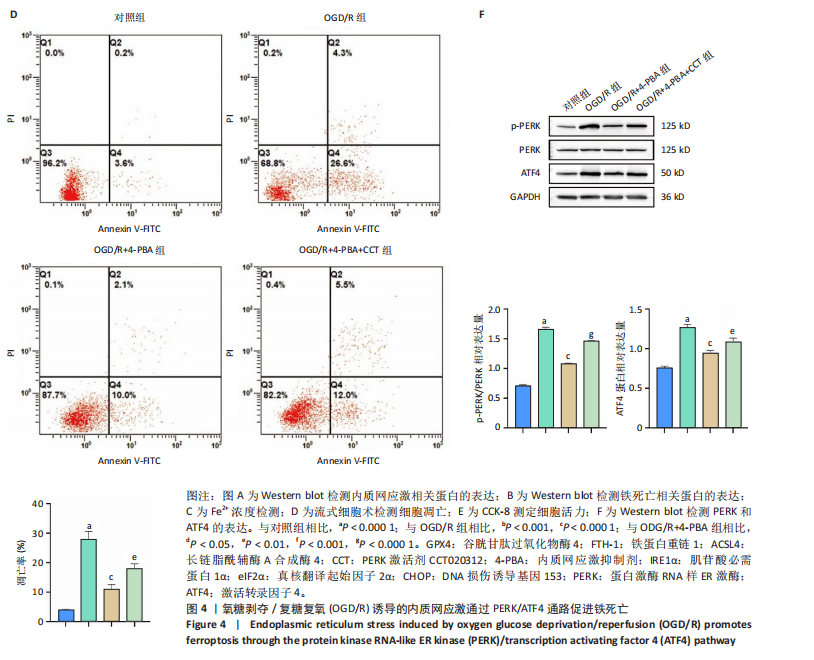
[1] SHEN XY, ZHANG XY, HAN PP, et al. Mechanisms of intermittent theta-burst stimulation attenuating nerve injury after ischemic reperfusion in rats through endoplasmic reticulum stress and ferroptosis. Mol Biol Rep. 2024;51(1):377.
[2] CHEN L, HUANG J, YAO ZM, et al. Procyanidins Alleviated Cerebral Ischemia/Reperfusion Injury by Inhibiting Ferroptosis via the Nrf2/HO-1 Signaling Pathway. Molecules. 2023;28(8):3582.
[3] WANG Q, XU S, WANG B, et al. Chemokine receptor 7 mediates miRNA-182 to regulate cerebral ischemia/reperfusion injury in rats. CNS Neurosci Ther. 2023;29(2):712-726.
[4] YU X, LUO Y, YANG L, et al. P‑hydroxybenzyl alcohol ameliorates neuronal cerebral ischemia‑reperfusion injury by activating mitochondrial autophagy through SIRT1. Mol Med Rep. 2023;27(3):68.
[5] LI X, MA N, XU J, et al. Targeting Ferroptosis: Pathological Mechanism and Treatment of Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021;2021:1587922.
[6] SHE R, LIU D, LIAO J, et al. Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: from pathology to therapeutic potential. Front Cell Neurosci. 2023; 17:1191629.
[7] LYU N, LI X. Sevoflurane Postconditioning Attenuates Cerebral Ischemia-Reperfusion Injury by Inhibiting SP1/ACSL4-Mediated Ferroptosis. Hum Exp Toxicol. 2023;42:9603271231160477.
[8] STOCKWELL BR, FRIEDMANN ANGELI JP, BAYIR H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273-285.
[9] PAN B, SUN J, LIU Z, et al. Longxuetongluo Capsule protects against cerebral ischemia/reperfusion injury through endoplasmic reticulum stress and MAPK-mediated mechanisms. J Adv Res. 2021;33:215-225.
[10] WANG L, LIU Y, ZHANG X, et al. Endoplasmic Reticulum Stress and the Unfolded Protein Response in Cerebral Ischemia/Reperfusion Injury. Front Cell Neurosci. 2022;16:864426.
[11] LI Y, LI M, FENG S, et al. Ferroptosis and endoplasmic reticulum stress in ischemic stroke. Neural Regen Res. 2024;19(3):611-618.
[12] LIU S, ZHANG X, YAO X, et al. Mammalian IRE1α dynamically and functionally coalesces with stress granules. Nat Cell Biol. 2024;26(6): 917-931.
[13] ZHANG Q, LIU G, LIU R, et al. Dual role of endoplasmic reticulum stress-ATF-6 activation in autophagy and apoptosis induced by cyclic stretch in myoblast. Apoptosis. 2023;28(5-6):796-809.
[14] MANDULA JK, CHANG S, MOHAMED E, et al. Ablation of the endoplasmic reticulum stress kinase PERK induces paraptosis and type I interferon to promote anti-tumor T cell responses. Cancer Cell. 2022;40(10):1145-1160.e9.
[15] LEE YS, LEE DH, CHOUDRY HA, et al. Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-talk between Ferroptosis and Apoptosis. Mol Cancer Res. 2018;16(7):1073-1076.
[16] WU Y, FAN X, CHEN S, et al. Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia-Reperfusion Injury via the PERK-ATF4-CHOP Pathway. Int J Mol Sci. 2022;24(1):544.
[17] GAO R, KALATHUR RKR, COTO-LLERENA M, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13(12):e14351.
[18] CUI J, LIU Y, CHANG X, et al. Acetaldehyde Induces Neurotoxicity In Vitro via Oxidative Stress- and Ca2+ Imbalance-Mediated Endoplasmic Reticulum Stress. Oxid Med Cell Longev. 2019;2019: 2593742.
[19] HAN Y, YUAN M, GUO YS, et al. Mechanism of Endoplasmic Reticulum Stress in Cerebral Ischemia. Front Cell Neurosci. 2021;15:704334.
[20] LV S, GENG X, YUN HJ, et al. Phenothiazines reduced autophagy in ischemic stroke through endoplasmic reticulum (ER) stress-associated PERK-eIF2α pathway. Exp Neurol. 2023;369:114524.
[21] WANG YH, LIAO JM, CHEN KM, et al. Lumbrokinase regulates endoplasmic reticulum stress to improve neurological deficits in ischemic stroke. Neuropharmacology. 2022;221:109277.
[22] LI HQ, XIA SN, XU SY, et al. γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress. Oxid Med Cell Longev. 2021;2021: 2961079.
[23] CHEN G, LI L, TAO H. Bioinformatics Identification of Ferroptosis-Related Biomarkers and Therapeutic Compounds in Ischemic Stroke. Front Neurol. 2021;12:745240.
[24] LIU C, LI Z, XI H. Bioinformatics analysis and in vivo validation of ferroptosis-related genes in ischemic stroke. Front Pharmacol. 2022;13:940260.
[25] LIU C, WANG G, HAN W, et al. Ferroptosis: a potential therapeutic target for stroke. Neural Regen Res. 2024;19(5):988-997.
[26] WANG L, LIU C, WANG L, et al. Astragaloside IV mitigates cerebral ischaemia-reperfusion injury via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis. Eur J Pharmacol. 2023;944:175516.
[27] CHEN X, YANG T, LUO Y, et al. Methodological and reporting quality evaluation of Buyang Huanwu decoction for experimental cerebral ischemia-reperfusion injury: a systematic review. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(5):831-849.
[28] LIU Z, NAN P, GONG Y, et al. Endoplasmic reticulum stress-triggered ferroptosis via the XBP1-Hrd1-Nrf2 pathway induces EMT progression in diabetic nephropathy. Biomed Pharmacother. 2023;164:114897.
[29] LI W, LI W, LENG Y, et al. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020;39(2):210-225.
[30] HUI Z, WANG S, LI J, et al. Compound Tongluo Decoction inhibits endoplasmic reticulum stress-induced ferroptosis and promoted angiogenesis by activating the Sonic Hedgehog pathway in cerebral infarction. J Ethnopharmacol. 2022;283:114634.
[31] ZENG T, ZHOU Y, YU Y, et al. rmMANF prevents sepsis-associated lung injury via inhibiting endoplasmic reticulum stress-induced ferroptosis in mice. Int Immunopharmacol. 2023;114:109608.
[32] HAN Y, SHEN XY, GAO ZK, et al. Pre-ischaemic Treatment with Enriched Environment Alleviates Acute Neuronal Injury by Inhibiting Endoplasmic Reticulum Stress-dependent Autophagy and Apoptosis. Neuroscience. 2023;513:14-27. |