Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (11): 2795-2805.doi: 10.12307/2026.069
Previous Articles Next Articles
Protective effect and mechanism of Zhigancao Decoction on doxorubicin-induced myocardial injury
Yu Manya1, Cui Xing2
- 1The First School of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 2Cancer Center, the Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250001, Shandong Province, China
-
Received:2025-02-06Accepted:2025-05-11Online:2026-04-18Published:2025-09-05 -
Contact:Cui Xing, MD, Chief physician, Professor, Doctoral supervisor, Cancer Center, the Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250001, Shandong Province, China -
About author:Yu Manya, The First School of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
Supported by:the National Natural Science Foundation of China (General Program), Nos. 82074348 and 82274491 (both to CX); the Joint Fund for Innovation and Development of Shandong Province Natural Science Foundation, No. ZR2023LZL009 (to CX)
CLC Number:
Cite this article
Yu Manya, Cui Xing. Protective effect and mechanism of Zhigancao Decoction on doxorubicin-induced myocardial injury[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2795-2805.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
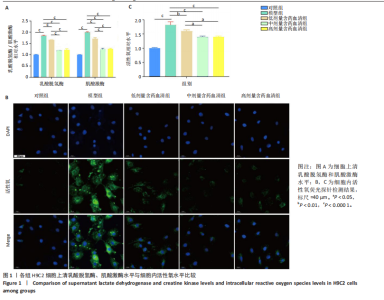
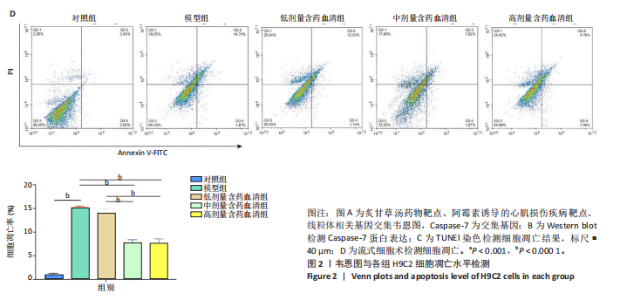
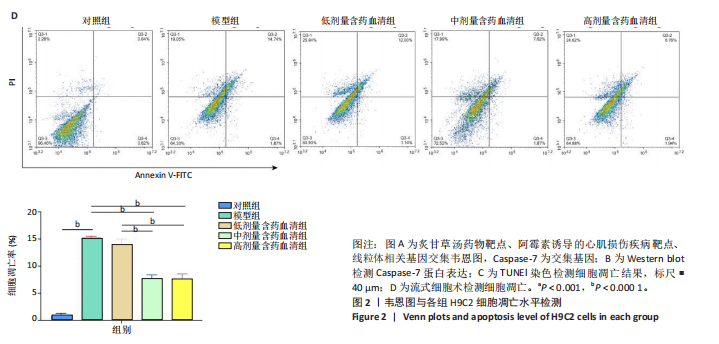
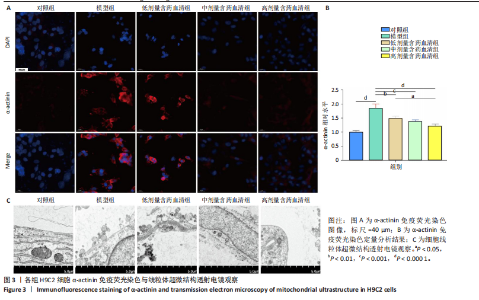
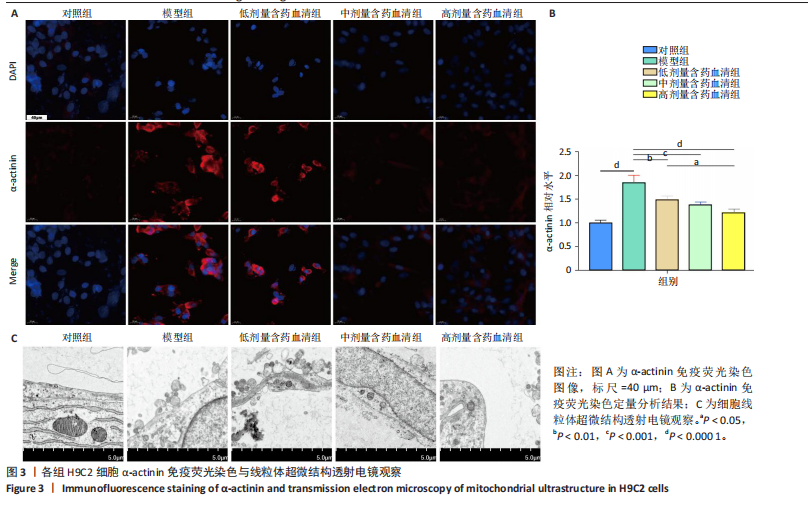
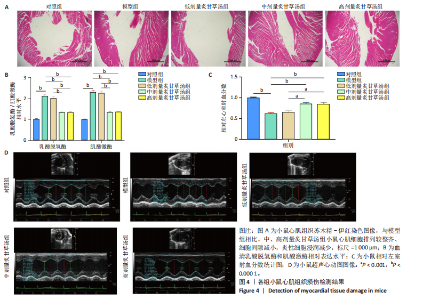
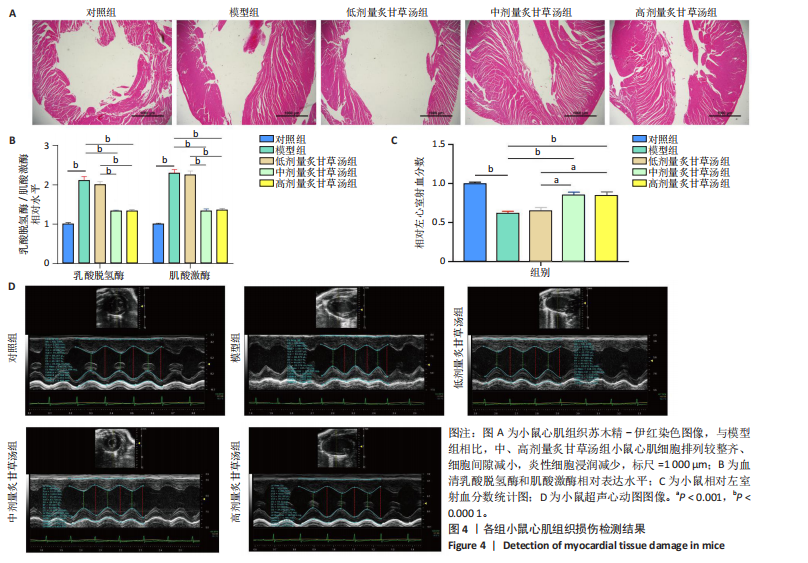
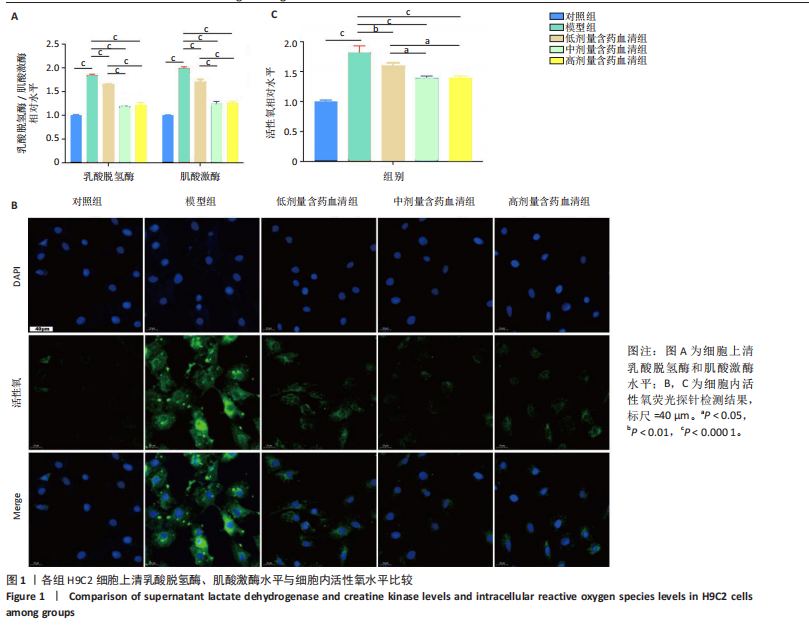
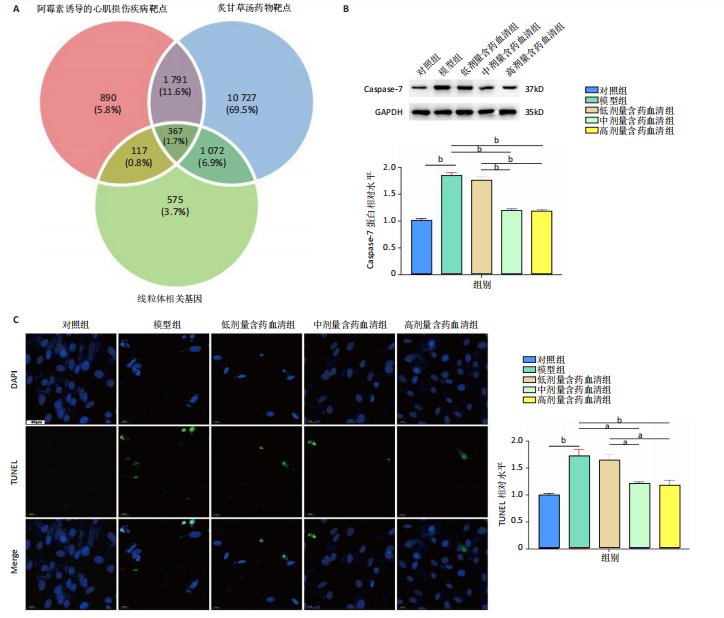
2.1 炙甘草汤减轻阿霉素诱导的心肌细胞损伤和氧化应激 与对照组相比,模型组细胞上清乳酸脱氢酶和肌酸激酶水平升高(P < 0.000 1);与模型组比较,低、中、高剂量含药血清组细胞上清乳酸脱氢酶和肌酸激酶水平降低(P < 0.000 1);与低剂量含药血清组比较,中、高剂量含药血清组细胞上清乳酸脱氢酶和肌酸激酶水平降低(P < 0.000 1);中、高剂量含药血清组细胞上清乳酸脱氢酶和肌酸激酶水平比较差异无显著性意义(P > 0.05),见图1A。 模型组细胞内活性氧水平高于对照组(P < 0.000 1),低、中、高剂量含药血清组细胞内活性氧水平低于模型组(P < 0.01或P < 0.000 1),中、高剂量含药血清组细胞内活性氧水平低于低剂量含药血清组(P < 0.05),中、高剂量含药血清组细胞内活性氧水平比较差异无显著性意义(P > 0.05),见图1B,C。 2.2 炙甘草汤减轻阿霉素诱导的心肌细胞凋亡 炙甘草汤药物靶点、疾病靶点、线粒体相关基因韦恩图显示,Caspase-7为交集基因,见图2A。Western blot检测结果显示,模型组凋亡效应子Caspase-7蛋白表达高于对照组(P < 0.000 1),中、高剂量含药血清组Caspase-7蛋白表达低于模型组、低剂量含药血清组(P < 0.000 1),中、高剂量含药血清组Caspase-7蛋白表达比较差异无显著性意义(P > 0.05),见图2B。TUNEL染色及流式细胞术检测结果显示,模型组细胞凋亡率高于对照组(P < 0.000 1),中、高剂量含药血清组细胞凋亡率低于模型组、低剂量含药血清组(P < 0.000 1或P < 0.001),中、高剂量含药血清组细胞凋亡率比较差异无显著性意义(P > 0.05),见图2C,D。 2.3 炙甘草汤减轻阿霉素诱导的心肌细胞肥大和线粒体损伤 免疫荧光染色结果显示,模型组α-actinin表达高于对照组(P < 0.000 1),低、中、高剂量含药血清组α-actinin表达低于模型组(P < 0.01,P < 0.001,P < 0.000 1),高浓度含药血清组α-actinin表达低于低剂量含药血清组(P < 0.05),中、高剂量含药血清组α-actinin表达比较差异无显著性意义(P > 0.05),低、中剂量含药血清组α-actinin表达比较差异无显著性意义(P > 0.05),见图3A,B。透射电镜下可见模型组线粒体形状不规则,呈现空泡化,线粒体嵴断裂、缺失,低、中、高剂量含药血清组线粒体结构逐渐恢复,其中高剂量含药血清组线粒体结构接近正常(图3C)。 2.4 炙甘草汤减轻阿霉素小鼠模型心肌损伤 30只小鼠全部进入结果分析。苏木精-伊红染色显示,与对照组相比,模型组小鼠心肌纤维排列紊乱、细胞间隙扩张,伴有大量炎性细胞浸润;与模型组相比,中、高剂量炙甘草汤组小鼠心肌细胞排列较整齐、细胞间隙减小,炎性细胞浸润减少(图4A)。 与对照组相比,模型组小鼠血清乳酸脱氢酶和肌酸激酶水平升高(P < 0.000 1);与模型组、低剂量炙甘草汤组相比,中、高剂量炙甘草汤组小鼠血清乳酸脱氢酶和肌酸激酶水平下降(P < 0.000 1),中、高剂量组血清乳酸脱氢酶和肌酸激酶水平比较差异无显著性意义(P > 0.05),见图4B。 超声心动图显示,模型组小鼠左室射血分数低于对照组(P < 0.000 1),中、高剂量炙甘草汤组小鼠左室射血分数高于模型组、低剂量炙甘草汤组(P <"
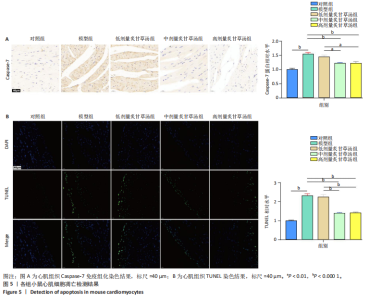
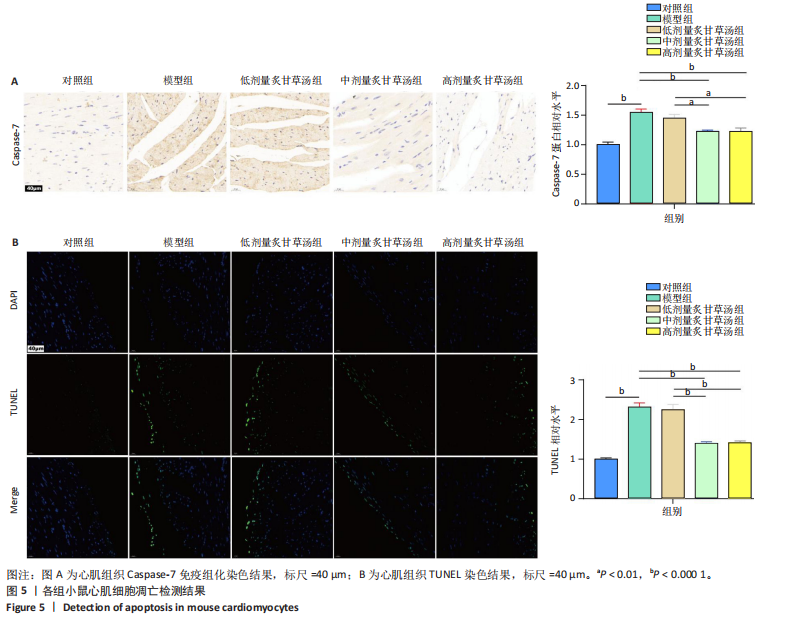
0.000 1或P < 0.001),中、高剂量炙甘草汤组小鼠左室射血分数比较差异无显著性意义(P > 0.05),见图4C,D。 2.5 炙甘草汤减轻阿霉素小鼠模型心肌凋亡 免疫组化染色结果显示,模型组心肌组织Caspase-7蛋白表达高于对照组(P < 0.000 1),中、高剂量炙甘草汤组Caspase-7蛋白表达低于模型组、低高剂量炙甘草汤组(P < 0.0001或P < 0.01),中、高剂量炙甘草汤组Caspase-7蛋白表达比较差异无显著性意义(P > 0.05),见图5A。 TUNEL染色结果显示,模型组心肌细胞凋亡率高于对照组(P < 0.000 1),中、高剂量炙甘草汤组心肌细胞凋亡率低于模型组、低高剂量炙甘草汤组(P < 0.000 1),中、高剂量炙甘草汤组心肌细胞凋亡率比较差异无显著性意义(P > 0.05),见图5B。"
| [1] MILLER KD, NOGUEIRA L, DEVASIA T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409-436. [2] DEMPKE W, ZIELINSKI R, WINKLER C, et al. Anthracycline-induced cardiotoxicity - are we about to clear this hurdle? Eur J Cancer. 2023; 185:94-104. [3] CAMILLI M, CIPOLLA CM, DENT S, et al. Anthracycline Cardiotoxicity in Adult Cancer Patients: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024;6(5):655-677. [4] SWAIN SM, WHALEY FS, EWER MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879. [5] ELFADADNY A, RAGAB RF, HAMADA R, et al. Natural bioactive compounds-doxorubicin combinations targeting topoisomerase II-alpha: Anticancer efficacy and safety. Toxicol Appl Pharmacol. 2023; 461:116405. [6] TAI P, CHEN X, JIA G, et al. WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J Transl Med. 2023;21(1):823. [7] WU BB, LEUNG KT, POON EN. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int J Mol Sci. 2022;23(3):1912. [8] HENRIKSEN PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971-977. [9] WU L, WANG L, DU Y, et al. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol Sci. 2023;44(1):34-49. [10] KITAKATA H, ENDO J, IKURA H, et al. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis. Int J Mol Sci. 2022;23(3):l1414. [11] 丁纯志,陈群,赵景胜.炙甘草汤防治阿霉素心脏毒性的疗效观察[J].北京中医药大学学报,2001,24(3):59-60. [12] ZHOU J, LIU Y, WEI X, et al. Glycnsisitin A: A promising bicyclic peptide against heart failure that facilitates TFRC-mediated uptake of iron in cardiomyocytes. Acta Pharm Sin B. 2024;14(7):3125-3139. [13] 张仲景.伤寒论[M].王叔和,钱超尘,郝万山整理,北京:人民卫生出版社,2005:2. [14] FU J, CHENG L, ZHANG J, et al. Isoliquiritin targeting m5C RNA methylation improves mitophagy in doxorubicin-induced myocardial cardiotoxicity. Phytomedicine. 2024;136:156293. [15] GAO F, LIANG T, LU YW, et al. Reduced Mitochondrial Protein Translation Promotes Cardiomyocyte Proliferation and Heart Regeneration. Circulation. 2023;148(23):1887-1906. [16] YU M, CAI Z, ZHANG J, et al. Aberrant NSUN2-mediated m5C modification of exosomal LncRNA MALAT1 induced RANKL-mediated bone destruction in multiple myeloma. Commun Biol. 2024;7(1):1249. [17] WALLACE KB, SARDAO VA, OLIVEIRA PJ. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ Res. 2020;126(7):926-941. [18] SANGWENI NF, GABUZA K, HUISAMEN B, et al. Molecular insights into the pathophysiology of doxorubicin-induced cardiotoxicity: a graphical representation. Arch Toxicol. 2022;96(6):1541-1550. [19] ARRIGONI R, JIRILLO E, CAIATI C. Pathophysiology of Doxorubicin-Mediated Cardiotoxicity. Toxics. 2025;13(4):277. [20] AL-MAAMARI A, SULTAN M, WU S, et al. Activation of sigma 1 receptor attenuates doxorubicin-induced cardiotoxicity by alleviating oxidative stress, mitochondria dysfunction, ER stress-related apoptosis, and autophagy impairment. Int J Biol Macromol. 2025;310(Pt 4):143549. [21] SONG L, QIU Q, JU F, et al. Mechanisms of doxorubicin-induced cardiac inflammation and fibrosis; therapeutic targets and approaches. Arch Biochem Biophys. 2024;761:110140. [22] ZHANG S, LIU X, BAWA-KHALFE T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11): 1639-1642. [23] KALYANARAMAN B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biol. 2020;29:101394. [24] WU L, SOWERS JR, ZHANG Y, et al. Targeting DNA damage response in cardiovascular diseases: from pathophysiology to therapeutic implications. Cardiovasc Res. 2023;119(3):691-709. [25] 杨洁文,徐叶峰,严卿莹.炙甘草汤干预阿霉素致大鼠心肌损害的能量代谢研究[J].浙江中医杂志,2021,56(4):258-260. [26] DUTTA B, LOO S, KAM A, et al. Cell-Permeable Microprotein from Panax Ginseng Protects Against Doxorubicin-Induced Oxidative Stress and Cardiotoxicity. Antioxidants (Basel). 2025;14(4):493. [27] KONG CY, GUO Z, SONG P, et al. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int J Biol Sci. 2022;18(2):760-770. [28] RAWAT PS, JAISWAL A, KHURANA A, et al. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708. [29] WANG X, LIU Z, LIN C. Metal ions-induced programmed cell death: how does oxidative stress regulate cell death? Life Sci. 2025;374:123688. [30] DAN DUNN J, ALVAREZ LA, ZHANG X, et al. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015;6:472-485. [31] SONGBO M, LANG H, XINYONG C, et al. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41-48. [32] TANAKA Y, NAGOSHI T, YOSHII A, et al. Xanthine oxidase inhibition attenuates doxorubicin-induced cardiotoxicity in mice. Free Radic Biol Med. 2021;162:298-308. [33] YANG L, GUAN J, LUO S, et al. Angiotensin IV ameliorates doxorubicin-induced cardiotoxicity by increasing glutathione peroxidase 4 and alleviating ferroptosis. Toxicol Appl Pharmacol. 2023;479:116713. [34] WU S, LAN J, LI L, et al. Sirt6 protects cardiomyocytes against doxorubicin-induced cardiotoxicity by inhibiting P53/Fas-dependent cell death and augmenting endogenous antioxidant defense mechanisms. Cell Biol Toxicol. 2023;39(1):237-258. [35] WANG T, XING G, FU T, et al. Role of mitochondria in doxorubicin-mediated cardiotoxicity: from molecular mechanisms to therapeutic strategies. Int J Med Sci. 2024;21(5):809-816. [36] WENNINGMANN N, KNAPP M, ANDE A, et al. Insights into Doxorubicin-induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol Pharmacol. 2019;96(2):219-232. [37] SCHIRONE L, D’AMBROSIO L, FORTE M, et al. Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay. Cells. 2022;11(13):2000. [38] AHMAD N, ULLAH A, CHU P, et al. Doxorubicin induced cardio toxicity through sirtuins mediated mitochondrial disruption. Chem Biol Interact. 2022;365:110028. [39] SUN Y, XIAO L, CHEN L, et al. Doxorubicin-Induced Cardiac Remodeling: Mechanisms and Mitigation Strategies. Cardiovasc Drugs Ther. 2025. doi: 10.1007/s10557-025-07673-6. [40] CHRISTIDI E, BRUNHAM LR. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12(4):339. [41] KITAKATA H, ENDO J, IKURA H, et al. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis. Int J Mol Sci. 2022;23(3):1414. [42] YANG H, LI S, LI W, et al. Actinomycin D synergizes with Doxorubicin in triple-negative breast cancer by inducing P53-dependent cell apoptosis. Carcinogenesis. 2024;45(4):262-273. [43] ARIES A, PARADIS P, LEFEBVRE C, et al. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101(18):6975-6980. [44] LI S, LIU H, LIN Z, et al. Isoorientin attenuates doxorubicin-induced cardiac injury via the activation of MAPK, Akt, and Caspase-dependent signaling pathways. Phytomedicine. 2022;101:154105. [45] DAS J, GHOSH J, MANNA P, et al. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem Pharmacol. 2011;81(7):891-909. [46] AN J, LI P, LI J, et al. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J Mol Med (Berl). 2009;87(4):401-410. [47] LAMKANFI M, KANNEGANTI TD. Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biol. 2010; 42(1):21-24. [48] CHRISTGEN S, TWEEDELL RE, KANNEGANTI TD. Programming inflammatory cell death for therapy. Pharmacol Ther. 2022;232:108010. [49] SAHOO G, SAMAL D, KHANDAYATARAY P, et al. A Review on Caspases: Key Regulators of Biological Activities and Apoptosis. Mol Neurobiol. 2023;60(10):5805-5837. |
| [1] | Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053. |
| [2] |
Dong Chunyang, Zhou Tianen, Mo Mengxue, Lyu Wenquan, Gao Ming, Zhu Ruikai, Gao Zhiwei.
Action mechanism of metformin combined with Eomecon chionantha Hance dressing in treatment of deep second-degree burn wounds#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2001-2013.
|
| [3] | Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807. |
| [4] | Cao Xinyan, Yu Zifu, Leng Xiaoxuan, Gao Shiai, Chen Jinhui, Liu Xihua. Effect of repetitive transcranial magnetic stimulation and transcranial direct current stimulation on motor function and gait in children with cerebral palsy: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1539-1548. |
| [5] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [6] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [7] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [8] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [9] | Zhou Xinying, Sun Xinyue, Zhu Wenhao. Insulin-like growth factors and ischemic stroke: a genome-wide association analysis in European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2909-2919. |
| [10] | Lu Yongheng, Zhu Shuang, Zhao Feiyan, Ai Fujun, Liu Yanjie, Dong Yangting, Guan Zhizhong, Wei Na. Effect of fluoride exposure on endoplasmic reticulum-mitochondrial calcium transfer and apoptosis in primary nerve cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 111-119. |
| [11] | Wu Zhijing, Li Jiali, Zhang Jiaxin, Wang Tangrong, Zheng Yuzhou, Sun Zixuan. Alpha-ketoglutarate engineered small extracellular vesicles delay skin aging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 120-129. |
| [12] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [13] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [14] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [15] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||