Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (7): 1858-1868.doi: 10.12307/2026.117
Previous Articles Next Articles
Secretome of stem cells from human exfoliated deciduous teeth: a new hotspot in tissue engineering and stem cell therapy
Liu Xingyu1, 2, Li Lijie1, 2
- 1Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, Inner Mongolia Autonomous Region, China; 2School of Stomatology, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China
-
Received:2025-03-01Revised:2025-06-20Accepted:2025-07-03Online:2026-03-08Published:2025-08-21 -
Contact:Li Lijie, MS, Chief physician, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, Inner Mongolia Autonomous Region, China; School of Stomatology, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China -
About author:Liu Xingyu, Master candidate, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, Inner Mongolia Autonomous Region, China; School of Stomatology, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China
CLC Number:
Cite this article
Liu Xingyu, Li Lijie. Secretome of stem cells from human exfoliated deciduous teeth: a new hotspot in tissue engineering and stem cell therapy[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1858-1868.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
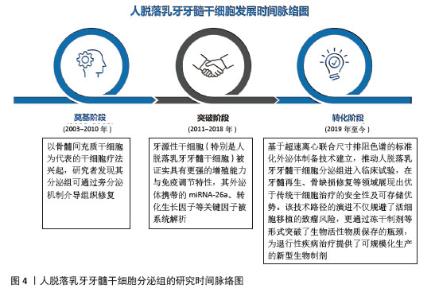
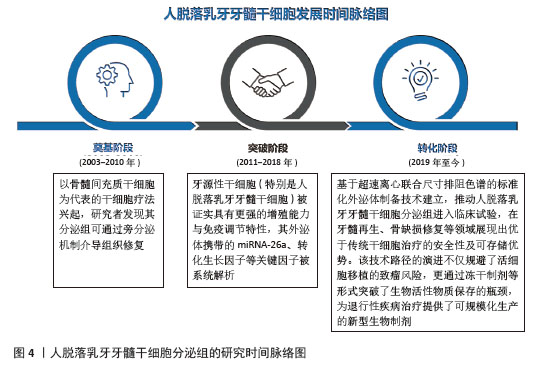
2.1 人脱落乳牙牙髓干细胞分泌组的研究时间脉络图 近年来,随着再生医学从传统细胞治疗向无细胞治疗策略的转型,人脱落乳牙牙髓干细胞分泌组研究取得了突破性进展,这一领域的发展可概括为3个阶段:奠基阶段(2003-2010年):以骨髓间充质干细胞为代表的干细胞疗法兴起,研究者发现其分泌组可通过旁分泌机制介导组织修复;突破阶段(2011-2018年):牙源性干细胞被证实具有更强的增殖能力与免疫调节特性,其外泌体携带的关键因子(如miRNA-26等)被系统解析;转化阶段(2019年至今):基于超速离心联合尺寸排阻色谱的标准化外泌体制备技术建立,推动人脱落乳牙牙髓干细胞分泌组进入临床试验,在牙髓再生、骨缺损修复等领域展现出优于传统干细胞治疗的安全性及可存储优势。该技术路径的演进不仅规避了活细胞移植的致瘤风险,更通过冻干制剂等形式突破了生物活性物质保存的瓶颈,为退行性疾病治疗提供了可规模化的新型生物制剂。见图4。"
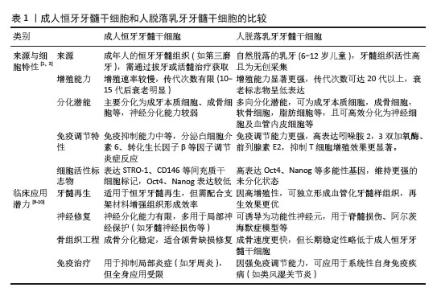
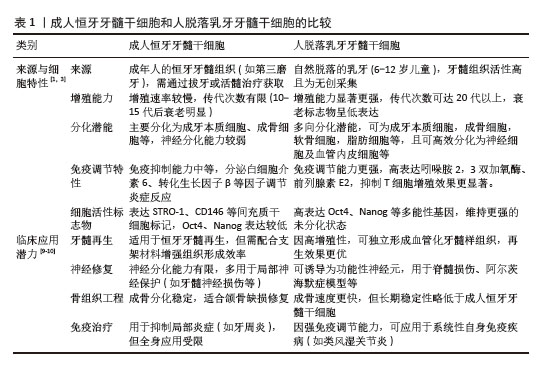
2.2 人脱落乳牙牙髓干细胞 人脱落乳牙牙髓干细胞于2003年由MIURA等[5]发现并从人脱落乳牙牙髓中提取,是一种来源于胚胎神经嵴外胚层的具有多向分化潜能的间充质干细胞。在体外,人脱落乳牙牙髓干细胞呈扁平、细长或成纤维细胞样形状。人脱落乳牙牙髓干细胞可分化成不同类型的细胞,如成牙本质细胞、成骨细胞、软骨细胞、脂肪细胞、内皮细胞、肌细胞及神经元,因此,人脱落乳牙牙髓干细胞成为口腔再生医学领域又一可靠细胞来源。与牙髓干细胞相比,人脱落乳牙牙髓干细胞可从脱落的乳牙牙髓中提取,无创且伦理争议较少,用于自体移植时,免疫排斥反应较少发生[6];人脱落乳牙牙髓干细胞的组织样本来源属于医疗废弃物,易于分离和提取;人脱落乳牙牙髓干细胞在液氮中冷冻保存2年以上仍能保持最初的干细胞特性,具有长期的基因组稳定性[7],是一种独特且极具潜力的干细胞群体。从再生医学的角度看,随着年龄增长,干细胞的数量和质量均会下降,而相对于牙髓干细胞,人脱落乳牙牙髓干细胞较接近胚胎特征,体外扩增速度快,多系分化能力类似于牙髓间充质干细胞,甚至更胜一筹[8]。人脱落乳牙牙髓干细胞来源于乳牙牙髓组织,与牙髓间充质干细胞相比,具有更高的增殖率。见表1[1,3,9-10]。 此外,人脱落乳牙牙髓干细胞含有丰富的营养物质如成纤维细胞生长因子、转化生长因子β、结缔组织生长因子、神经生长因子、骨形态发生蛋白等,它们能更好地提高人脱落乳牙牙髓干细胞的增殖能力和功能,因此人脱落乳牙牙髓干细胞在多能性和增殖能力方面具有优势[11]。人脱落乳牙牙髓干细胞还可以抑制肿瘤细胞的增殖,调节免疫功能,减少炎症,促进伤口愈合[12-13]。研究表明,在一定的诱导条件下,人脱落乳牙牙髓干细胞可表达多种神经元细胞标记物(巢蛋白、谷氨酸脱酸酶和神经元特异性核蛋白)[14]。一些研究发现人脱落乳牙牙髓干细胞中存在肝细胞特异性标志物,能够分化为肝细胞[15]。因此,人脱落乳牙牙髓干细胞在免疫系统疾病、神经系统疾病、肝脏疾病、骨缺损、皮肤创伤和口腔疾病治疗中显示出广泛的应用前景。"
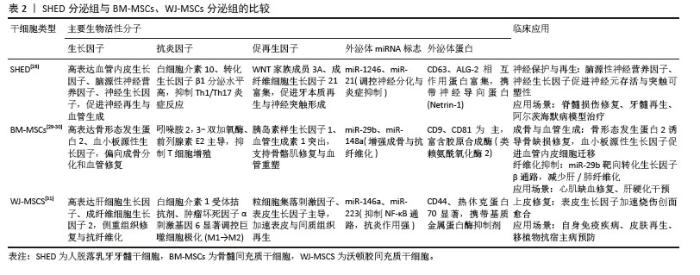
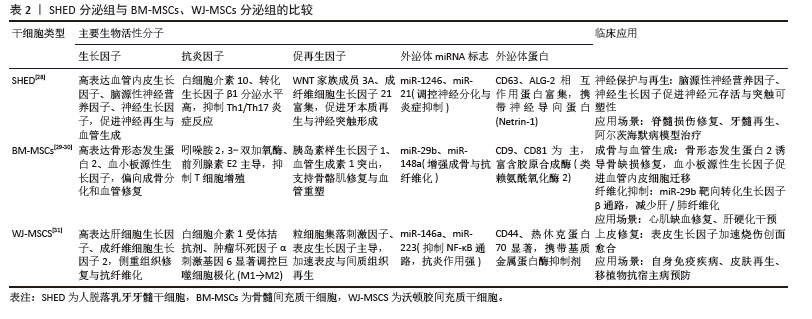
2.3 间充质干细胞分泌组 虽然间充质干细胞在再生性疾病和炎症性疾病有一定治疗作用,但以间充质干细胞为基础的治疗存在如移植后可能分化为其他非目标细胞类型、发挥过度的免疫抑制、病毒传播风险、微环境缺氧和营养缺乏等一系列不可忽视的安全性问题[16]。此外,同种异体移植物的免疫排斥反应和潜在的致癌风险,以及干细胞保存程序和干细胞培养方法的复杂性,都需要进一步验证。研究表明,间充质干细胞不是通过干细胞取代受损细胞来发挥治疗作用,而是通过旁分泌生物活性物质来发挥治疗作用,即输注的间充质干细胞通过释放外泌体等生物活性物质,这些物质作用于靶细胞,从而改变靶细胞的功能,促进靶细胞的再生活性[17]。与基于干细胞的治疗相比,干细胞衍生分泌组的优势之一是易于长时间保存、灭菌、包装,且不会破坏其生物学特性,而且可以大量生产,无需侵入性提取程序,减少了对供体的伤害风险[18-19]。 间充质干细胞分泌组包括可溶性蛋白(如生长因子、趋化因子、抗体、酶、黏附分子、受体、激素、抗菌肽等)、游离核酸、脂质和细胞外囊泡[20]。细胞外囊泡包括微囊泡(100-1 000 nm)、外泌体(40-100 nm)和凋亡小体(1-5 μm)[21-23]。微囊泡和外泌体是膜结合颗粒,可以发挥旁分泌和内分泌作用[24]。这些分泌蛋白组可以在干细胞培养的条件培养基中获取。因此,含有分泌蛋白组的条件培养基可用于干细胞再生疗法。 SHIN等[25]的一项研究调查了不同的间充质干细胞来源分泌组特征,分别是:骨髓间充质干细胞、脂肪间充质干细胞、胎盘间充质干细胞和脐带间充质干细胞,该研究在脂肪间充质干细胞分泌组中鉴定出265个蛋白,在骨髓间充质干细胞分泌组中鉴定出253个蛋白,在胎盘间充质干细胞分泌组中鉴定出511个蛋白,在脐带间充质干细胞分泌组中鉴定出440个蛋白。研究表明,这些蛋白在细胞发育、迁移和代谢活动等多种生物学功能中发挥着显著作用。虽然间充质干细胞的起源不同,但它们的分泌组表现出相似的功能特性,突出了间充质干细胞来源分泌组在再生医学和组织工程领域的潜在应用。 2.4 人脱落乳牙牙髓干细胞分泌组 与其他间充质干细胞的分泌组相比,KONALA等[26]的研究表明,人脱落乳牙牙髓干细胞衍生的分泌组在减少瘢痕形成、血管生成、炎症、纤维化和免疫调节方面优于骨髓间充质干细胞[27]。 该研究还分析了人脱落乳牙牙髓干细胞、骨髓间充质干细胞和脐带间充质干细胞的分泌组,发现人脱落乳牙牙髓干细胞衍生的分泌组比骨髓间充质干细胞和脐带间充质干细胞衍生的分泌组具有更高的血管生成素1、血小板源性生长因子、白细胞介素10、基质细胞衍生因子1、肝细胞生长因子、血管内皮生长因子1、成纤维细胞生长因子2和γ干扰素表达水平。此外,研究还发现,人脱落乳牙牙髓干细胞衍生的分泌组比骨髓间充质干细胞衍生的分泌组含有更多的白细胞介素6,表明源自人脱落乳牙牙髓干细胞的分泌组含有更广泛的生物活性分子,可以促进组织再生和修复。因此,可以认为人脱落乳牙牙髓干细胞是再生医学和口腔医学中无细胞疗法的又一可靠来源。见表2[28-31]。"
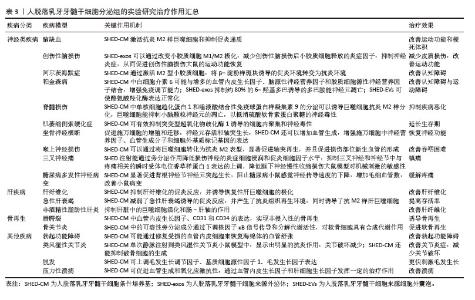
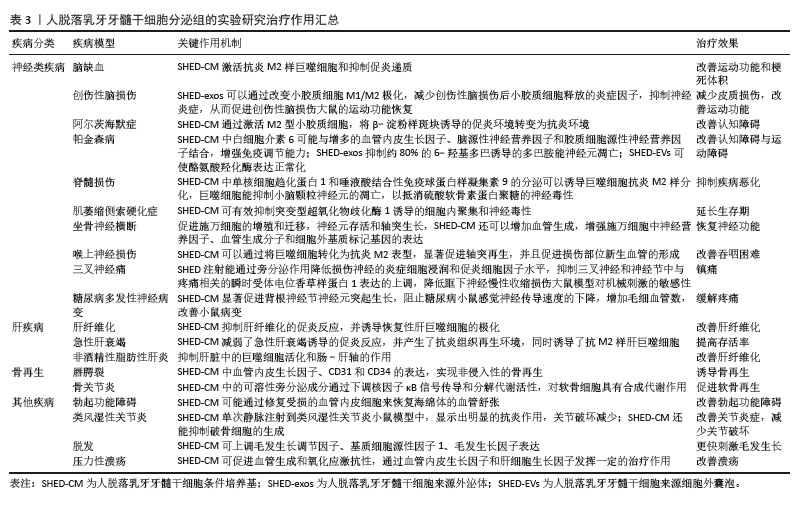
2.5 人脱落乳牙牙髓干细胞分泌组的临床疾病治疗研究 研究表明,人脱落乳牙牙髓干细胞衍生的分泌组在牙组织再生、神经系统疾病、肝疾病以及其他一些疾病的治疗方面均有一定作用。 2.5.1 人脱落乳牙牙髓干细胞分泌组在牙齿组织再生中的治疗作用 目前临床大量实践证实,虽根管治疗仍为牙髓疾病的普遍治疗方法,但存在一定局限性,如虽能清除感染,但无法恢复牙髓活力及牙本质再生能力,导致牙齿脆性增加;另一方面,受生物材料的限制,目前仍无法重建功能性牙髓-牙本质复合体。基于此,学者们将研究目光聚焦于干细胞及组织工程技术,旨在实现牙髓-牙本质复合体的再生。人脱落乳牙牙髓干细胞具有上述特点及优势,成为牙髓再生的“种子细胞”之一。 GUO等[32]研究显示,在脱细胞牙基质和脱落乳牙牙髓干细胞聚合体共培养条件下产生的外泌体,能显著提高成牙本质相关蛋白如牙本质涎磷蛋白(dentin sialophosphoprotein,DSPP)和牙本质基质蛋白4(dentin matrix protein 4,DMP4)的表达水平,有利于牙本质的形成。WU等[33]的研究表明,脱细胞牙基质和脱落乳牙牙髓干细胞聚合体来源的外泌体产量约为普通细胞来源外泌体的3倍,且能更明显地促进人脱落乳牙牙髓干细胞的内皮分化;该外泌体中表达上调的miR-26a,可通过转化生长因子β/SMAD2/3信号传导,参与调控血管生成。XUAN等[34]研究发现,使用自体乳牙牙髓干细胞治疗因创伤导致的年轻恒牙牙髓坏死,可观察到含有正常成牙本质细胞层的牙髓组织再生,经过2年时间的随访观察,发现再生的牙髓仍然保持牙髓活性。LAN等[35]研究表明,体内或背部皮下植入异位牙根后,将人脱落乳牙牙髓干细胞分泌组注入实验模型中,可通过miR-26a激活转化生长因子β/Smad2/3信号通路,促进牙本质-牙髓复合物再生和血管生成。GAO等[36]通过蛋白质组学分析证实,低氧可诱导人脱落乳牙牙髓干细胞产生更多的外泌体,且低氧条件下产生的外泌体可通过黏着斑信号通路及血管内皮生长因子信号通路促进血管生成。此外,YUAN等[37]实验研究表明,将载有人脱落乳牙牙髓干细胞的辛伐他汀功能化甲基丙烯酸明胶微球注射至实验模型中,能够诱导富含血管的牙髓样组织再生。上述大量研究均表明,人脱落乳牙牙髓干细胞分泌组在牙本质再生、牙髓再生及牙髓牙本质复合体再生方面均有巨大潜力。 2.5.2 人脱落乳牙牙髓干细胞分泌组对神经系统疾病的治疗作用 脑缺血是一类神经脑血管疾病,所引起的脑供血和供氧中断是世界范围内死亡和残疾的重要原因[38]。研究表明,鼻腔内给予人脱落乳牙牙髓干细胞条件培养基可以促进神经元祖细胞向梗死区迁移分化,诱导血管生成,改善永久性大脑中动脉闭塞大鼠模型的运动功能和梗死体积。与骨髓间充质干细胞移植相比,人脱落乳牙牙髓干细胞条件培养基减少了梗死体积。此外,人脱落乳牙牙髓干细胞条件培养基可促进内源性神经祖细胞的迁移和分化,促进血管生成,改善缺血性脑损伤。 创伤性脑损伤是全世界所有年龄段死亡和致残的主要原因之一[39]。小胶质细胞介导的神经炎症在创伤性脑损伤中起着关键作用[40]。研究证明,人脱落乳牙牙髓干细胞外泌体可以通过改变小胶质细胞M1/M2极化,减少小胶质细胞释放的炎症因子,抑制神经炎症,从而促进创伤性脑损伤大鼠的运动功能恢复。在进一步的研究中,人脱落乳牙牙髓干细胞外泌体以剂量依赖性的方式降低了促炎小胶质细胞M1表型细胞标记物,激活了M2小胶质细胞,从而通过抗炎细胞因子来抑制神经炎症,这些结果在体内得到了进一步证实,在创伤性脑损伤大鼠模型中,人脱落乳牙牙髓干细胞外泌体改善了大鼠运动功能,减少了皮质损伤[41]。 阿尔茨海默症是一类常见的神经退行性疾病,临床表现为进行性记忆丧失、神经精神症状和日常行动功能障碍[42],其主要病理特征为脑萎缩、淀粉样斑块、脑内神经原纤维缠结[43]。MITA等[44]研究了人脱落乳牙牙髓干细胞条件培养基在阿尔茨海默症小鼠模型中的治疗效果,以鼻内给予人脱落乳牙牙髓干细胞条件培养基的方式,证实其可使小鼠认知功能显著改善。人脱落乳牙牙髓干细胞条件培养基含有多种神经再生机制相关的因子,如神经保护、轴突伸长、神经传递、炎症抑制和小胶质细胞的调节。研究证明人脱落乳牙牙髓干细胞条件培养基能够通过激活抗炎的M2型小胶质细胞,将β -淀粉样斑块诱导的促炎环境转变为抗炎环境,同时保护初级大脑神经元免受促炎环境下产生的谷氨酸神经毒性的影响,改善小鼠的认知缺陷。 帕金森病是第二大最常见的老年神经退行性疾病,也是最常见的运动障碍,临床表现为静息性震颤、运动缓慢、僵直和姿势不稳[45]。研究显示,人脱落乳牙牙髓干细胞分泌的血管内皮生长因子和脑源性神经营养因子浓度增高,可能起到神经保护的作用,而人脱落乳牙牙髓干细胞条件培养基中白细胞介素6的浓度显著升高,可与其他因子协同增强免疫调节能力,以改善帕金森病大鼠的脑损伤[46]。人脱落乳牙牙髓干细胞条件培养基包含的活性因子可能通过调节胆碱能突触、钙信号通路、5-羟色胺能突触和轴突引导,使纹状体中酪氨酸羟化酶水平升高,黑质和纹状体中α -突触核蛋白水平降低,显著改善鱼藤酮诱导的帕金森病症状。 脊髓损伤是严重的中枢神经系统损伤,临床症状包括损伤水平以下的运动感觉以及肌功能障碍[47]。损伤脊髓附近的反应性星形胶质细胞和少突胶质细胞分别产生硫酸软骨素蛋白多糖和髓鞘相关糖蛋白,这些细胞外分子作为轴突生长抑制剂,协同加速脊髓损伤后的进展性恶化[48-49]。研究表明,将人脱落乳牙牙髓干细胞条件培养基注射到脊髓损伤的大鼠脊髓鞘内,可显著促进功能恢复,而且人脱落乳牙牙髓干细胞条件培养基中单核细胞趋化蛋白1和唾液酸结合性免疫球蛋白样凝集素9可以诱导巨噬细胞抗炎M2样分化,巨噬细胞能扩展神经突并抑制小脑颗粒神经元的凋亡,以抵消硫酸软骨素蛋白多糖的神经毒性[50]。 肌萎缩侧索硬化症又称渐冻症,其特征是上、下运动神经元选择性和进行性变性和丧失。干细胞疗法是治疗肌萎缩侧索硬化症的一种有前景的治疗方法[51],这种疗法可以通过营养支持和免疫调节影响各种致病机制,有可能减缓疾病进展。研究表明,人脱落乳牙牙髓干细胞条件培养基含有血管内皮生长因子和肝细胞生长因子等,这些成分对包括肌萎缩侧索硬化症在内的神经系统疾病有一定有效性,其水平远高于骨髓源性干细胞分泌组[52]。另外一项研究表明,人脱落乳牙牙髓干细胞条件培养基可有效抑制突变型超氧化物歧化酶1诱导的细胞内聚集和神经毒性,并对肌萎缩侧索硬化症患者诱导多能干细胞衍生的运动神经元具有保护作用[53]。此外,将人脱落乳牙牙髓干细胞条件培养基应用于肌萎缩侧索硬化症小鼠模型,可以延长小鼠的生存期[54]。 因此,人脱落乳牙牙髓干细胞条件培养基对于肌萎缩侧索硬化症患者来说是一种有前景的治疗选择。 坐骨神经横断体外实验证明,人脱落乳牙牙髓干细胞条件培养基中的神经营养因子能够促进施万细胞的增殖和迁移、神经元存活和轴突生长。研究发现,人脱落乳牙牙髓干细胞条件培养基还可以增加血管生成,增强施万细胞中神经营养因子、血管生成分子和细胞外基质标记基因的表达;研究认为,人脱落乳牙牙髓干细胞条件培养基中的多种生物活性因子可能是以协同或叠加的方式促进周围神经再生,减少肌萎缩,从而显著恢复坐骨神经横断模型大鼠的神经功能[55]。 在另一项喉上神经损伤相关研究中发现人脱落乳牙牙髓干细胞条件培养基可以通过将巨噬细胞转化为抗炎M2表型,显著促进轴突再生,并且促进损伤部位新生血管的形成,改善喉上神经损伤小鼠的吞咽困难[56]。 原发性三叉神经痛是指无明确原因引起的三叉神经感觉根分布区阵发性疼痛,为临床常见病、多发病[57],首选卡马西平药物治疗。研究表明,将人脱落乳牙牙髓干细胞注射到原发性三叉神经痛大鼠模型后,能通过旁分泌作用降低损伤神经的炎症细胞浸润和促炎细胞因子水平,抑制三叉神经和神经节中与疼痛相关的瞬时受体电位香草样蛋白1表达的上调,降低眶下神经慢性收缩损伤大鼠对机械刺激的敏感性[58],达到镇痛的效果。 糖尿病多发性神经病变是糖尿病常见的并发症之一,发病率高达60%[59],表现为足部麻木和/或糖尿病周围神经性疼痛。糖尿病多发性神经病变的主要病理改变为有髓纤维数目明显减少、轴索变性显著和神经束膜增厚[60]。研究表明,人脱落乳牙牙髓干细胞条件培养基能通过细胞因子促进背根神经节神经元突起生长,显著阻止糖尿病多发性神经病变小鼠感觉神经传导速度的下降,增加毛细血管数量[61]。上述研究说明人脱落乳牙牙髓干细胞条件培养基可以通过参与组织再生、增加局部血流量和给予神经营养保护,缓解糖尿病所引起的神经症状。 2.5.3 人脱落乳牙牙髓干细胞分泌组对肝疾病的治疗作用 肝纤维化是病毒性肝炎、自身免疫性肝炎和酒精性肝病等引起的持续性慢性肝病的结果[62]。严重的肝纤维化导致不可逆转的肝硬变和随后的肝功能衰竭。目前,治疗终末期肝纤维化最有效的治疗方法是肝移植,然而,移植供体供不应求。HIRATA等[63]的研究表明,人脱落乳牙牙髓干细胞条件培养基可抑制肝纤维化的促炎反应,并诱导恢复性肝巨噬细胞的极化。人脱落乳牙牙髓干细胞条件培养基除了通过刺激肝星状细胞凋亡来改善肝纤维化外,还可以抑制促炎递质(如肿瘤坏死因子α、白细胞介素1β和诱导型一氧化氮合酶)的基因表达,引起活化的肝星状细胞凋亡。因此,合理猜测人脱落乳牙牙髓干细胞条件培养基可通过多方面的作用改善肝纤维化。 在急性肝衰竭中,自身免疫反应过度会导致肝脏严重损伤,从而引发全身炎症反应、进行性多器官衰竭并最终导致猝死。尽管肝脏本身具有一定的组织修复活性,但原位肝移植是目前急性肝衰竭唯一的治疗方法。ITO等[64]研究发现将人脱落乳牙牙髓干细胞或人脱落乳牙牙髓干细胞条件培养基单次静脉注射到D-半乳糖胺诱导的急性肝衰竭大鼠模型中,可明显提高肝脏受损动物的存活率。输注的人脱落乳牙牙髓干细胞的植入率非常低,但二者均发挥相似水平的治疗效果,表明人脱落乳牙牙髓干细胞通过旁分泌机制作用于急性肝衰竭。该研究表明,人脱落乳牙牙髓干细胞条件培养基减弱了急性肝衰竭诱导的促炎反应,并产生了抗炎组织再生环境,同时诱导了抗炎M2样肝巨噬细胞。 非酒精性脂肪性肝炎及其引起的肝纤维化和肝硬化发病率逐年上升,MUTO等[65]通过重复静脉注射人脱落乳牙牙髓干细胞条件培养基至非酒精性脂肪性肝炎小鼠模型中,发现小鼠外周血中肝酶水平显著下降,显著改善了肝纤维化和炎症表现,研究观察到注射人脱落乳牙牙髓干细胞条件培养基后的非酒精性脂肪性肝炎小鼠肝脏中促炎性和促纤维化递质(如肿瘤坏死因子α、转化生长因子β和趋化因子2)的基因表达降低。此外,研究发现人脱落乳牙牙髓干细胞条件培养基可促进γ干扰素和肿瘤坏死因子α诱导的人结直肠腺癌细胞单层功能障碍的恢复,同时抑制肝脏中内毒素受体4的基因表达。研究表明,人脱落乳牙牙髓干细胞条件培养基除了对肝细胞的保护作用和诱导具有独特抗炎表型的巨噬细胞外,还可能通过肠-肝轴抑制非酒精性脂肪性肝炎导致的纤维化。 2.5.4 人脱落乳牙牙髓干细胞分泌组对骨再生的治疗作用 人脱落乳牙牙髓干细胞有良好的成骨能力,研究发现,人脱落乳牙牙髓干细胞条件培养基在体外可促进种植体表面磷酸钙盐和胞外基质蛋白的沉积,进而促进骨髓基质细胞的黏附;在体内可刺激种植体周围新骨形成[66]。HIRAKI等[67]研究结果表明,人脱落乳牙牙髓干细胞条件培养基能够实现非侵入性的骨再生,有望重建唇腭裂患者的牙槽骨缺损。MUHAMMAD等[68]研究发现,人脱落乳牙牙髓干细胞条件培养基增强了骨关节炎小鼠软骨细胞中聚集蛋白和Ⅱ型胶原的表达,其通过下调NF-κB信号传导促进软骨细胞的合成代谢。OGASAWARA等[69]研究证明人脱落乳牙牙髓干细胞条件培养基明显抑制了颞肌炎症,改善了髁状突软骨表面的光滑度。人脱落乳牙牙髓干细胞条件培养基可以通过机械应力抑制软骨降解、减轻炎症、促进细胞增殖、促进软骨再生并阻止颞下颌关节骨性关节炎的发生。人脱落乳牙牙髓干细胞条件培养基可能成为一种有效的组织再生治疗剂,用于治疗严重的颞下颌关节损伤患者。 2.5.5 人脱落乳牙牙髓干细胞分泌组对其他疾病的治疗作用 研究表明,超过80%的勃起功能障碍是由器质性改变引起的,血管源性疾病最为常见。近年来,在全球再生医学领域的基础研究中,已有多篇论文证明,干细胞在体外分泌的生物活性物质对于组织再生的作用比干细胞本身更为强大。KOGA等[70]将人脱落乳牙牙髓干细胞条件培养基直接注射到勃起功能障碍患者的海绵体内进行治疗及随访观察,从研究结果来看勃起功能显著改善,推测人脱落乳牙牙髓干细胞条件培养基可能通过修复受损的血管内皮细胞来恢复海绵体的血管舒张,从而促进内皮细胞产生一氧化氮。 类风湿性关节炎是以滑膜增生和慢性炎症为主要特征的慢性自身免疫性疾病,可导致关节内软骨和骨的进行性破坏。ISHIKAWA等[71]将人脱落乳牙牙髓干细胞条件培养基单次静脉注射到类风湿性关节炎小鼠模型中,显示出明显的抗炎作用,关节破坏减少,关节炎症状总体改善。因此,人脱落乳牙牙髓干细胞条件培养基有望成为一种新型的类风湿性关节炎治疗药物。 脱发是一种由过度脱发引起的临床病症,可能导致秃顶,其原因仍然难以捉摸。人脱落乳牙牙髓干细胞条件培养基在体内和体外治疗脱发方面也显示出有希望的结果。 GUNAWARDENA等[72]实验研究中,用剪刀剃除背部区域的小鼠模型分别皮下注射人人脱落乳牙牙髓干细胞条件培养基和人毛囊干细胞条件培养基,结果显示,与人毛囊干细胞条件培养基相比,人脱落乳牙牙髓干细胞条件培养基通过上调毛发生长调节因子、基质细胞源性因子1、毛发生长因子,能更快地刺激毛发生长。 压力性溃疡在全球范围内随着年龄的增长而增加,但没有有效的治疗方法。虽然间充质干细胞可以促进皮肤伤口愈合,但间充质干细胞条件培养基对缺血再灌注损伤诱导的皮肤压力性溃疡影响的研究甚少。KATAHIRA等[73]的研究表明,使用脱落乳牙牙髓干细胞条件培养基可通过促进血管生成和氧化应激抗性,通过血管内皮生长因子和肝细胞生长因子发挥一定的治疗作用。见表3。"
| [1] FRIEDENSTEIN AJ, CHAILAKHYAN RK, LATSINIK NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331-340. [2] GRONTHOS S, MANKANI M, BRAHIM J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625-13630. [3] CHALISSERRY EP, NAM SY, PARK SH, et al. Therapeutic potential of dental stem cells. J Tissue Eng. 2017;8:2041731417702531. [4] 万然,史旭,刘京松,等.间充质干细胞分泌组治疗脊髓损伤的研究进展[J].中国组织工程研究,2021,25(7):1088-1095. [5] MIURA M, GRONTHOS S, ZHAO M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807-5812. [6] REN H, SANG Y, ZHANG F, et al. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016;2016:3516574. [7] BHANDI S, ALKAHTANI A, MASHYAKHY M, et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J Pers Med. 2021;11(7):589. [8] NAKAMURA S, YAMADA Y, KATAGIRI W, et al. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35(11):1536-1542. [9] HUANG GT, SONOYAMA W, LIU Y, et al. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008; 34(6):645-651. [10] YAMAZA T, KENTARO A, CHEN C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5. [11] GIORDANO G, LA MONACA G, ANNIBALI S, et al. Stem cells from oral niches: a review. Ann Stomatol (Roma). 2011;2(1-2):3-8. [12] ANOOP M, DATTA I. Stem Cells Derived from Human Exfoliated Deciduous Teeth (SHED) in Neuronal Disorders: A Review. Curr Stem Cell Res Ther. 2021;16(5):535-550. [13] NOURBAKHSH N, SOLEIMANI M, TAGHIPOUR Z, et al. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol. 2011;55(2):189-195. [14] NUTI N, CORALLO C, CHAN BM, et al. Multipotent Differentiation of Human Dental Pulp Stem Cells: a Literature Review. Stem Cell Rev Rep. 2016;12(5):511-523. [15] KERKIS I, AMBROSIO CE, KERKIS A, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J Transl Med. 2008;6:35. [16] GLASSBERG MK, MINKIEWICZ J, TOONKEL RL, et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest. 2017;151(5):971-981. [17] LI JK, YANG C, SU Y, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury. Front Immunol. 2021;12: 684496. [18] BERMUDEZ MA, SENDON-LAGO J, EIRO N, et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest Ophthalmol Vis Sci. 2015;56(2): 983-992. [19] VIZOSO FJ, EIRO N, CID S, et al. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18(9):1852. [20] 李佳林,张耀东,娄艳茹,等.间充质干细胞分泌组发挥作用的分子机制[J].中国组织工程研究,2025,29(7):1512-1522. [21] KATAGIRI W, OSUGI M, KAWAI T, et al. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int J Oral Maxillofac Implants. 2013;28(4):1009-1016. [22] LEE Y, EL ANDALOUSSI S, WOOD MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012; 21(R1):R125-134. [23] RAPOSO G, STOORVOGEL W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383. [24] KIM KO, CHOI SM, KIM HS, et al. Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Eng Regen Med. 2013;10:93-101. [25] SHIN S, LEE J, KWON Y, et al. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int J Mol Sci. 2021;22(2):845. [26] KONALA VBR, BHONDE R, PAL R. Secretome studies of mesenchymal stromal cells (MSCs) isolated from three tissue sources reveal subtle differences in potency. In Vitro Cell Dev Biol Anim. 2020;56(9):689-700. [27] INOUE T, SUGIYAMA M, HATTORI H, et al. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2013;19(1-2):24-29. [28] NICOLA F, MARQUES MR, ODORCYK F, et al. Stem Cells from Human Exfoliated Deciduous Teeth Modulate Early Astrocyte Response after Spinal Cord Contusion. Mol Neurobiol. 2019; 56(1):748-760. [29] MARTINO MM, TORTELLI F, MOCHIZUKI M, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011; 3(100):100ra89. [30] NENG W, SHUANG-YING L, JUN X, et al. A ‘jump-to-coalescence’ mechanism during nanoparticle growth revealed by in situ aberration-corrected transmission electron microscopy observations. Nanotechnology. 2016;27(20):205605. [31] WANG J, ZHANG XR, HE WF, et al. Research advances on the mechanism of dendritic epidermal T lymphocytes in wound healing. Zhonghua Shao Shang Za Zhi. 2021;37(3): 296-300. [32] GUO H, LI B, WU M, et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials. 2021;279:121223. [33] WU M, LIU X, LI Z, et al. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 2021; 54(7):e13074. [34] XUAN K, LI B, GUO H, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455): eaaf3227. [35] LAN BY, LIN X, CHEN WJ, et al. Effect of lipopolysaccharide-stimulated exosomes from human dental pulp stem cells combined with stromal cell-derived factor-1 on dental pulp regeneration. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022; 57(1):60-67. [36] GAO Y, YUAN Z, YUAN X, et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact Mater. 2022;14: 377-388. [37] YUAN X, YUAN Z, WANG Y, et al. Vascularized pulp regeneration via injecting simvastatin functionalized GelMA cryogel microspheres loaded with stem cells from human exfoliated deciduous teeth. Mater Today Bio. 2022;13:100209. [38] KATAN M, LUFT A. Global Burden of Stroke. Semin Neurol. 2018;38(2):208-211. [39] MASEL BE, DEWITT DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529-1540. [40] KUMAR A, LOANE DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26(8):1191-1201. [41] LI Y, YANG YY, REN JL, et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8(1):198. [42] 杨泽若,张燚,胡柳,等.亚洲人阿尔兹海默症miRNA-mRNA网络的生物信息学分析[J].生物信息学,2022,20(1):46-55. [43] LIU PP, XIE Y, MENG XY, et al. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther. 2019;4:29. [44] MITA T, FURUKAWA-HIBI Y, TAKEUCHI H, et al. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav Brain Res. 2015;293:189-197. [45] MHYRE TR, BOYD JT, HAMILL RW, et al. Parkinson’s disease. Subcell Biochem. 2012;65:389-455. [46] ZHANG N, LU X, WU S, et al. Intrastriatal transplantation of stem cells from human exfoliated deciduous teeth reduces motor defects in Parkinsonian rats. Cytotherapy. 2018;20(5):670-686. [47] CHEN YR, LAI PL, CHIEN Y, et al. Improvement of Impaired Motor Functions by Human Dental Exfoliated Deciduous Teeth Stem Cell-Derived Factors in a Rat Model of Parkinson’s Disease. Int J Mol Sci. 2020;21(11):3807. [48] ZIMMER MB, NANTWI K, GOSHGARIAN HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007; 30(4):319-330. [49] YIU G, HE Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7(8): 617-627. [50] SAKAI K, YAMAMOTO A, MATSUBARA K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122(1):80-90. [51] MATSUBARA K, MATSUSHITA Y, SAKAI K, et al. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci. 2015;35(6):2452-2464. [52] ALJABRI A, HALAWANI A, BIN LAJDAM G, et al. The Safety and Efficacy of Stem Cell Therapy as an Emerging Therapy for ALS: A Systematic Review of Controlled Clinical Trials. Front Neurol. 2021;12:783122. [53] UEDA T, ITO T, INDEN M, et al. Stem Cells From Human Exfoliated Deciduous Teeth-Conditioned Medium (SHED-CM) is a Promising Treatment for Amyotrophic Lateral Sclerosis. Front Pharmacol. 2022; 13:805379. [54] WANG J, ZUZZIO K, WALKER CL. Systemic Dental Pulp Stem Cell Secretome Therapy in a Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci. 2019;9(7):165. [55] SUGIMURA-WAKAYAMA Y, KATAGIRI W, OSUGI M, et al. Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cells Dev. 2015; 24(22):2687-2699. [56] TSURUTA T, SAKAI K, WATANABE J, et al. Dental pulp-derived stem cell conditioned medium to regenerate peripheral nerves in a novel animal model of dysphagia. PLoS One. 2018;13(12):e0208938. [57] 冯保会,李世亭.原发性三叉神经痛治疗研究进展[J].中华神经外科疾病研究杂志,2009,8(3):280-282. [58] BAI X, ZHANG X, WANG C, et al. Stem Cells from Human Exfoliated Deciduous Teeth Attenuate Trigeminal Neuralgia in Rats. Stem Cells Int. 2021; 2021:8819884. [59] ZENKER J, ZIEGLER D, CHRAST R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36(8):439-449. [60] 董荣芳,张铭,郑丹枫,等.糖尿病周围神经病的病理学研究[J].诊断病理学杂志,2015,22(3):133-138. [61] MIURA-YURA E, TSUNEKAWA S, NARUSE K, et al. Secreted factors from cultured dental pulp stem cells promoted neurite outgrowth of dorsal root ganglion neurons and ameliorated neural functions in streptozotocin-induced diabetic mice. J Diabetes Investig. 2020;11(1):28-38. [62] ROEHLEN N, CROUCHET E, BAUMERT TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020;9(4):875. [63] HIRATA M, ISHIGAMI M, MATSUSHITA Y, et al. Multifaceted Therapeutic Benefits of Factors Derived From Dental Pulp Stem Cells for Mouse Liver Fibrosis. Stem Cells Transl Med. 2016;5(10):1416-1424. [64] ITO T, ISHIGAMI M, MATSUSHITA Y, et al. Secreted Ectodomain of SIGLEC-9 and MCP-1 Synergistically Improve Acute Liver Failure in Rats by Altering Macrophage Polarity. Sci Rep. 2017;7:44043. [65] MUTO H, ITO T, TANAKA T, et al. Conditioned medium from stem cells derived from human exfoliated deciduous teeth ameliorates NASH via the Gut-Liver axis. Sci Rep. 2021;11(1):18778. [66] OMORI M, TSUCHIYA S, HARA K, et al. A new application of cell-free bone regeneration: immobilizing stem cells from human exfoliated deciduous teeth-conditioned medium onto titanium implants using atmospheric pressure plasma treatment. Stem Cell Res Ther. 2015;6(1):124. [67] HIRAKI T, KUNIMATSU R, NAKAJIMA K, et al. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020; 26(2):381-390. [68] MUHAMMAD SA, NORDIN N, HUSSIN P, et al. Protective effects of stem cells from human exfoliated deciduous teeth derived conditioned medium on osteoarthritic chondrocytes. PLoS One. 2020;15(9):e0238449. [69] OGASAWARA N, KANO F, HASHIMOTO N, et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthritis Cartilage. 2020;28(6):831-841. [70] KOGA S, HORIGUCHI Y. Efficacy of a cultured conditioned medium of exfoliated deciduous dental pulp stem cells in erectile dysfunction patients. J Cell Mol Med. 2022; 26(1):195-201. [71] ISHIKAWA J, TAKAHASHI N, MATSUMOTO T, et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone. 2016;83:210-219. [72] GUNAWARDENA TNA, MASOUDIAN Z, RAHMAN MT, et al. Dental derived stem cell conditioned media for hair growth stimulation. PLoS One. 2019;14(5): e0216003. [73] KATAHIRA Y, MURAKAMI F, INOUE S, et al. Protective effects of conditioned media of immortalized stem cells from human exfoliated deciduous teeth on pressure ulcer formation. Front Immunol. 2023;13:1010700. |
| [1] | Zhu Jing, Zhai Xiguo, Wu Qizhen, Wang Yupei, Lyu Ling, Hou Qinzheng. Development and application of human amniotic membrane in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1818-1827. |
| [2] | Yuan Xiaoshuang, Yang Xu, Yang Bo, Chen Xiaoxu, Tian Ting, Wang Feiqing, Li Yanju, Liu Yang, Yang Wenxiu. Effect of conditioned medium of diffuse large B-cell lymphoma cells on proliferation and apoptosis of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1632-1640. |
| [3] | Cui Lianxu, Li Haomin, Xu Junrong, Tan Baodong, Lu Dahong, Peng Siwei, Wang Jinhui. Effect of umbilical cord mesenchymal stem cell conditioned medium on tissue repair after traumatic craniocerebral injury in miniature pigs [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1730-1735. |
| [4] | Xu Haichao, Luo Lihua, Pan Yihuai. Application and progress of dental pulp stem cells and their derivatives in dental pulp regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 153-162. |
| [5] | Zhang Zhaowei, Chen Ouzile, Bai Mingru, Wang Chenglin. Therapeutic potential of bioactive substances secreted by dental mesenchymal stem cells for bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 163-174. |
| [6] | Luo Wenbin, Li Ruoyun, Pan Chaofan, Luo Changjiang. Engineered exosomes for repairing tissue damage: application potential, excellent biological stability, and targeting specificity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 204-217. |
| [7] | Ma Wenjing, Zhang Jinyu, Jiang Mingxia, Xiu Bingshui, Bai Rui, Liu Yuhan, Chen Xuyi, Yuan Zengqiang, Liu Zhiqiang. Scaffold-free three-dimensional human umbilical cord mesenchymal stem cell secretome repairs mouse skin injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 68-77. |
| [8] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [9] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [10] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [11] | Liu Chengyuan, Guo Qianping. Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7490-7498. |
| [12] | Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu. Decellularized tendon scaffold: a biomedical material for tendon injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7385-7392. |
| [13] | Gang Fangli, Dang Zhongxiu, Li Ruiyun, Li Xiao, Sun Xiaoyang. Techniques and performance of biominerals for fabricating bone tissue engineering scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 5957-5967. |
| [14] | Yang Na, Liu Yang, Hao Huiqin. Molecular mechanism of adipose tissue-derived mesenchymal stem cells in treatment of acute liver injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 5041-5050. |
| [15] | Long Chenyan, Cheng Biao, Tian Ju. Cellular and molecular mechanisms of platelet-rich plasma in promoting wound healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2793-2801. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||