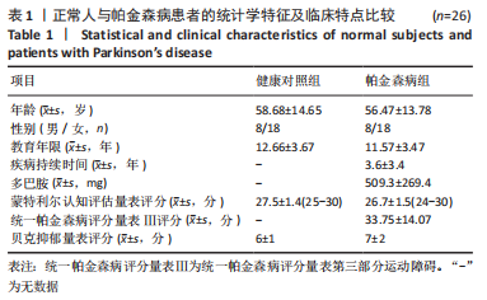
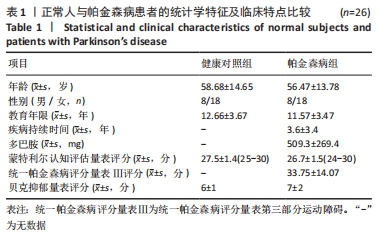
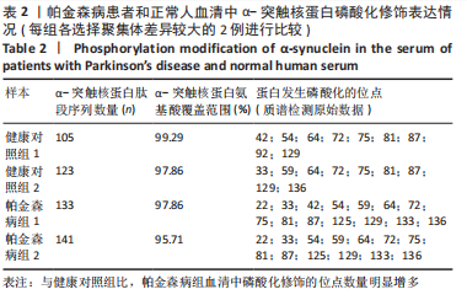
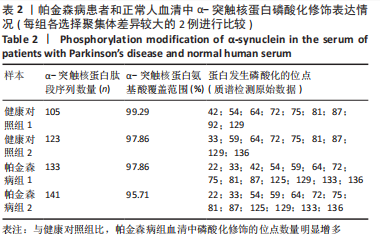
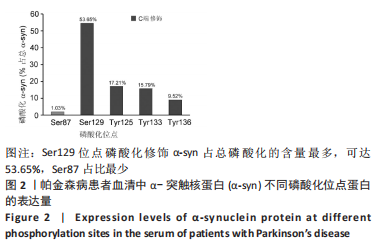
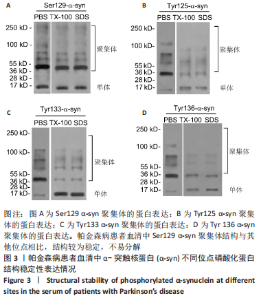
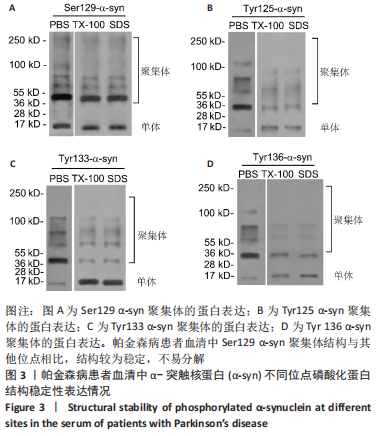
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (35): 5577-5582.doi: 10.12307/2024.205
Phosphorylation modification of alpha synuclein in serum of patients with Parkinson’s disease
Qi Xue1, Li Jiahui1, Zhu Yuanfeng1, Yu Lu1, Wang Peng1, 2
- 1Department of Human Anatomy, 2Laboratory of Neurodegenerative Diseases, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China
-
Received:2023-03-10Accepted:2023-03-20Online:2023-12-18Published:2023-06-01 -
Contact:Wang Peng, MD, Associate professor, Master’s supervisor, Department of Human Anatomy, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China; Laboratory of Neurodegenerative Diseases, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China -
About author:Qi Xue, Master candidate, Department of Human Anatomy, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China -
Supported by:Natural Science Foundation of Jilin Province, No. YDZJ202201ZYTS575 (to WP); the Health Department of Jilin Province, No. 2018J083 (to WP); Postgraduate Innovative Project of Beihua University, No. 2021017 (to QX)
CLC Number:
Cite this article
Qi Xue, Li Jiahui, Zhu Yuanfeng, Yu Lu, Wang Peng. Phosphorylation modification of alpha synuclein in serum of patients with Parkinson’s disease[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5577-5582.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] HAYES MT. Parkinson’s Disease and Parkinsonism. Am J Med. 2019; 132(7):802-807. [2] GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18(5):459-480. [3] WEINTRAUB D, MAMIKONYAN E. The neuropsychiatry of Parkinson disease: a perfect storm. Am J Geriatr Psychiatry. 2019;27(9):998-1018. [4] ZHENGYU S, SHUAI L, XIYU L, et al. Prevalence of Parkinson’s disease in adults aged 65 years and older in china: a multicenter population-based survey. Neuroepidemiology. 2022;56(1):50-58. [5] YU RC, PARK SJ, SANG MP. Molecular events underlying the cell‐to‐cell transmission of α‐synuclein. The FEBS Journal. 2021;288(23):6593-6602. [6] BRAAK H, DEL TREDICI K, RÜB U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2): 197-211. [7] CHAN PY, RIPIN ZM, HALIM SA, et al. Motion characteristics of subclinical tremors in Parkinson’s disease and normal subjects. Sci Rep. 2022;12(1):4021. [8] SIMONET C, SCHRAG A, LEESs AJ, et al. The motor prodromes of parkinson’s disease: from bedside observation to large-scale application. J Neurol. 2021;268(6):2099-2108. [9] MAROTEAUX L, CAMPANELLI JT, SCHELLER RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8(8):2804-2815. [10] SORRENTINO ZA, HASS E, VIJAYAYAGHAVAN N, et al. Corrigendum to “Carboxy-terminal truncation and phosphorylation of α-synuclein elongates survival in a prion-like seeding mouse model of synucleinopathy” [Neurosci. Lett. 732 (2020) 1-11/135017]. Neurosci Lett. 2022;783:136719. [11] PERINAN MT, BROLIN K, BANDRES-CIGA S, et al. Effect modification between genes and environment and Parkinson’s disease risk. Ann Neurol. 2022;92(5):715-724. [12] KAWAHATA I, FINKELSTEIN DI, FUKUNAGA K. Pathogenic impact of α-synuclein phosphorylation and its kinases in α-synucleinopathies. Int J Mol Sci. 2022;23(11):6216. [13] 安俊言,安思训,王鹏.帕金森病患者血清polo样激酶2活性升高促进α-突触核蛋白聚集[J].吉林医药学院学报,2019,40(3):171-174. [14] MOORS TE, MONA D, LUEHE S, et al. Multi-platform quantitation of alpha-synuclein human brain proteoforms suggests disease-specific biochemical profiles of synucleinopathies. Acta Neuropathol Commun. 2022;10(1):1-22. [15] 中华医学会神经病学分会帕金森病及运动障碍学组,中国医师协会神经内科医师分会帕金森病及运动障碍专业.中国帕金森病的诊断标准(2016版)[J].中华神经科杂志,2016,49(4):268-271. [16] HASIN D, HATENBUEHLER ML, KEYES K, et al. Substance use disorders: Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10). Addiction. 2006;101(s1):59-75. [17] JANKOVIC J, TAN EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795-808. [18] CHOOG CJ, MOCHIZUKI H. Neuropathology of α-synuclein in Parkinson’s disease. Neuropathology. 2022;42(2):93-103. [19] BAEK MS, LEE MJ, KIM HK, et al. Temporal trajectory of biofluid markers in Parkinson’s disease. Sci Rep. 2021;11:14820. [20] CHIU PY, YANG FC, CHIU MJ, et al. Relevance of plasma biomarkers to pathologies in Alzheimer’s disease, Parkinson’s disease and frontotemporal dementia. Sci Rep. 2022;12(1):17919. [21] FUJIWARA H, HASEGAWA M, DOHMAE N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2): 160-164. [22] YANG Y, SHI Y, SCHWEIGHAUSER M, et al. Structures of α-synuclein filaments from human brains with Lewy pathology. Nature. 2022; 610(7933):791-795. [23] SILVA-COSTA LC, SMITH BJ. Post-translational modifications in brain diseases: a future for biomarkers. Adv Exp Med Biol. 2022;1382:129-141. [24] WANG J, TAN Y, PENG Q, et al. Structural brain changes in Ser129-phosphorylated alpha-synuclein rats based on voxel-based morphometry. Behav Brain Res. 2020;393:112786. [25] 杨智源,吕春香,王鹏.重组人α-突触核蛋白原核表达系统的建立及纯化方法[J].中国老年学杂志,2011,31(9):1616-1618. [26] SANO K, IWASAKI Y, YAMASHITA Y, et al. Tyrosine 136 phosphorylation of α-synuclein aggregates in the Lewy body dementia brain: involvement of serine 129 phosphorylation by casein kinase 2. Acta Neuropathol Commun. 2021;9(1):182. [27] MA L, YANG C, ZHANG X, et al. C-terminal truncation exacerbates the aggregation and cytotoxicity of α-Synuclein: a vicious cycle in Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis. 2018; 1864(12):3714-3725. [28] MAROTTA NP, ARA J, Uemura N, et al. Alpha-synuclein from patient Lewy bodies exhibits distinct pathological activity that can be propagated in vitro. Acta Neuropathol Commun. 2021;9(1):1-18. [29] LASSEN LB, THOMSEN MS, BASSO E, et al. Mutation of tyrosine sites in the human alpha-synuclein gene induces neurotoxicity in transgenic mice with soluble alpha-synuclein oligomer formation. Cells. 2022; 11(22):3673. [30] WU W, SUNG CC, YU P, et al. Correction: S-Nitrosylation of G protein-coupled receptor kinase 6 and Casein kinase 2 alpha modulates their kinase activity toward alpha-synuclein phosphorylation in an animal model of Parkinson’s disease. PLoS One. 2020;15(6):e0235296. [31] KOSS DJ, ERSKINE D, PORTER A, et al. Nuclear alpha-synuclein is present in the human brain and is modified in dementia with Lewy bodies. Acta Neuropathol Commun. 2022;10(1):98. [32] DING J, WANG Y, HUANG J, et al. Role of alpha-synuclein phosphorylation at Serine 129 in methamphetamine-induced neurotoxicity in vitro and in vivo. NeuroReport. 2020;31(11):787-797. |
| [1] | Shan Jiaxin, Zhang Yilong, Wu Hongtao, Zhang Jiayuan, Li Anan, Liu Wengang, Xu Xuemeng, Zhao Chuanxi. Changes in muscle strength and pain in patients receiving Jianpi Yiqi Huoxue Formula after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1378-1382. |
| [2] | Lou Guo, Zhang Yan, Fu Changxi. Role of endothelial nitric oxide synthase in exercise preconditioning-induced improvement of myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1283-1288. |
| [3] | Qi Xue, Li Jiahui, Zhu Yuanfeng, Yu Lu, Wang Peng. Abnormal modification of alpha-synuclein and its mechanism in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1301-1306. |
| [4] | Shen Jiangyong, He Xi, Tang Yuting, Wang Jianjun, Liu Jinyi, Chen Yuanyuan, Wang Xinyi, Liu Tong, Sun Haoyuan. RAS-selective lethal small molecule 3 inhibits the fibrosis of pathological scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1168-1173. |
| [5] | Tan Nengxian, Wu Wenzheng, Zheng Churong, Luo Lieliang, Gu Peng, Ouyang Chongzhi, Zheng Xiaohui. Finite element analysis of different fixation methods of partially threaded cannulated screws for treating vertical femoral neck fractures [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 873-878. |
| [6] | Zheng Jiafa, Song Xiufeng, Li Hongzhi, Zhou Jinming, Guan Shengyi, Yu He. Open reduction and internal fixation via the para-Achilles tendon approach for the treatment of posterior malleolus sandwich fractures [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 934-938. |
| [7] | Zhou Xiaowen, Fu Zuchang, Huang Fei, Ai Jianguo, Zhao Feng. Bone defect blocked by bone cement segmental filling in single-plane tibial bone transport [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 736-740. |
| [8] | Bu Xianzhong, Bu Baoxian, Xu Wei, Zhang Chi, Zhang Yisheng, Zhong Yuanming, Li Zhifei, Tang Fubo, Mai Wei, Zhou Jinyan. Analysis of serum differential proteomics in patients with acute cervical spondylotic radiculopathy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 535-541. |
| [9] | Wu Tian, Zhao Yue, Hu Rong. Effect of nanobubbles carrying double antibodies on the proliferation of ovarian cancer cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 341-346. |
| [10] | Zhu Zhiqi, Yuan Sijie, Zhang Zilin, Ji Shijie, Meng Mingsong, Yan Anming, Han Jing. Mechanism underlying the effect of Liuwei Dihuang Pill on osteolysis and osteogenesis induced by titanium particles [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 392-397. |
| [11] | Gao Xueyu, Zhang Wentao, Sun Tianze, Zhang Jing, Li Zhonghai. Application of metal ions in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 439-444. |
| [12] | Xiao Yi, Lu Shuo, Ge Lite, Lu Ming. Interaction between autophagy and mesenchymal stem cells in treatment of neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3111-3116. |
| [13] | Chen Na, Wang Yanlin, Sun Huifang, Fan Feiyan, Li Donghong, Zhang Yunke. Shexiang Huangqi compound dripping pills-containing serum promotes proliferation and differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2960-2966. |
| [14] | Zhang Shuai, You Shengnan, Du Wenjing, Wang Lei, Xu Guizhi. Effects of transcranial magneto-acoustical stimulation on beta oscillations in neural circuits of healthy and Parkinson’s disease rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2519-2526. |
| [15] | Yuan Tianyi, Liu Hongjiang, Yang Zengqiang, Lu Xingbao, Maimaitiyibubaji, Zhou Zhiheng, Cui Yong. Identification of differences in N6-methyladenosine-related genes in steroid-induced femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2159-2165. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||