Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (20): 3151-3157.doi: 10.12307/2023.484
Previous Articles Next Articles
Neuroprotective effects of sodium butyrate and acetylation proteomics analysis in fluorosis rats
Li Yangjie1, Qi Rong2, Zhang Xinyu1, Cheng Jiaji3, Zhou Ning4, Cui Xue3, Cheng Shuang4, Wang Zhengdong5, Yan Nan6
- 1Basic Medical School, 2Science Experiment Center, 3School of Public Health, 4Medical Applied Technology School, 5Department of Anatomy, Basic Medical School, 6Department of Basic Rehabilitation, Medical Applied Technology School, Shenyang Medical College, Shenyang 110034, Liaoning Province, China
-
Received:2022-05-10Accepted:2022-08-05Online:2023-07-18Published:2022-11-19 -
Contact:Yan Nan, MD, Associate professor, Department of Basic Rehabilitation, Medical Applied Technology School, Shenyang Medical College, Shenyang 110034, Liaoning Province, China Wang Zhengdong, MD, Professor, Department of Anatomy, Basic Medical School, Shenyang Medical College, Shenyang 110034, Liaoning Province, China -
About author:Li Yangjie, Master candidate, Basic Medical School, Shenyang Medical College, Shenyang 110034, Liaoning Province, China -
Supported by:the National Natural Science Foundation of China (Youth Project), No. 81803200 (to YN); Liaoning Provincial Department of Education Scientific Research Project, No. SYYX202008 (to WZD); Social Governance Science and Technology Project of Shenyang Science and Technology Program, No. 21-108-9-11 (to YN); Shenyang Municipal Young and Middle-aged Talent Program for Scientific and Technological Innovation, No. RC200238 (to YN); Graduate Student Science and Technology Innovation Fund Project of Shenyang Medical College, No. Y20210503 (to LYJ)
CLC Number:
Cite this article
Li Yangjie, Qi Rong, Zhang Xinyu, Cheng Jiaji, Zhou Ning, Cui Xue, Cheng Shuang, Wang Zhengdong, Yan Nan. Neuroprotective effects of sodium butyrate and acetylation proteomics analysis in fluorosis rats[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3151-3157.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
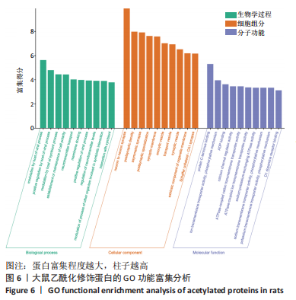
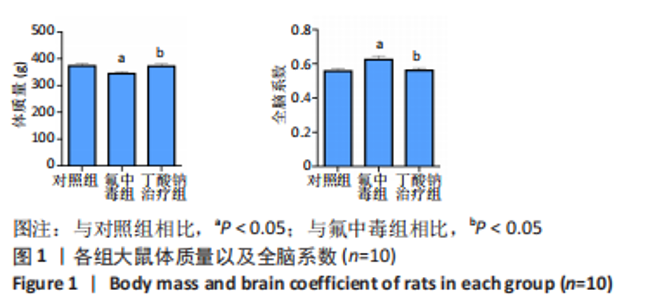
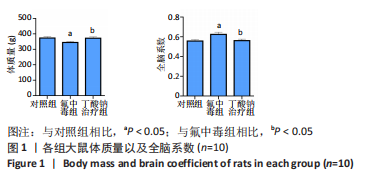
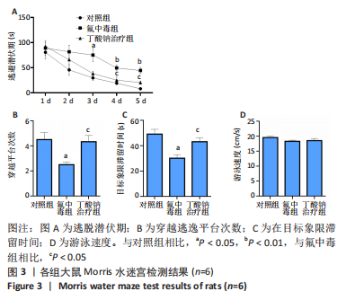
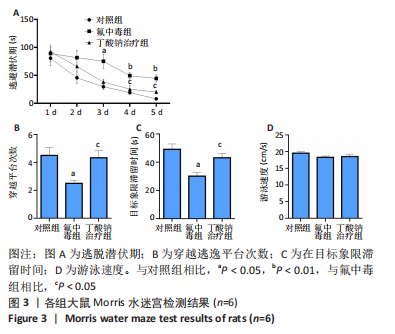
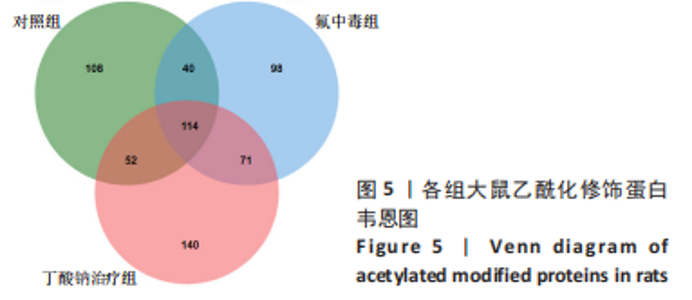
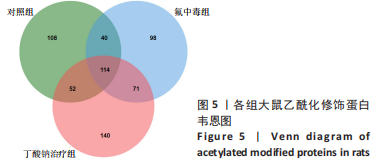
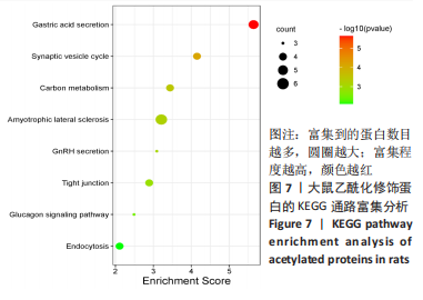
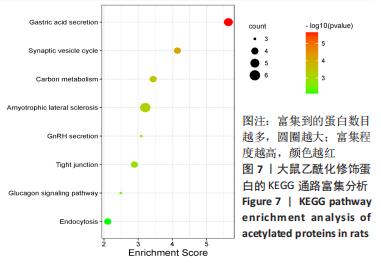
2.6 乙酰化修饰蛋白GO功能富集分析 将上述52个蛋白导入Gene Ontology数据库,并进行功能富集分析,GO分析包括生物学过程(BP)、分子功能(MF)和细胞组分(CC)3个部分,选取P值最大的前10个进行柱状图绘制。在生物学过程中,乙酰化修饰蛋白富集较为均匀,包括宿主与病毒相互作用、宿主对共生过程的调节、细胞极性的建立或维持、神经递质运输、神经递质水平的调节、膜对接等生物学过程。在细胞组分中,乙酰化修饰蛋白显著富集于突触功能,包括神经元间突触、突触后密度、不对称突触、突触后膜特化、突触膜、细胞外囊泡、囊泡运输、突触囊泡、Schaffer侧支至海马CA1区突触传递。在分子功能中,乙酰化修饰蛋白显著富集于跨膜物质转运如离子跨膜转运蛋白活性及其磷酸化机制、钙通道调节活性、ATP酶偶联离子跨膜转运体活性、钠与钾跨膜转运蛋白活性及其磷酸化机制等,见图6。"

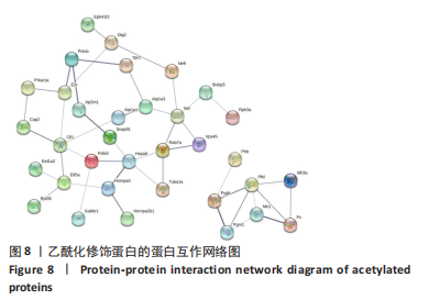
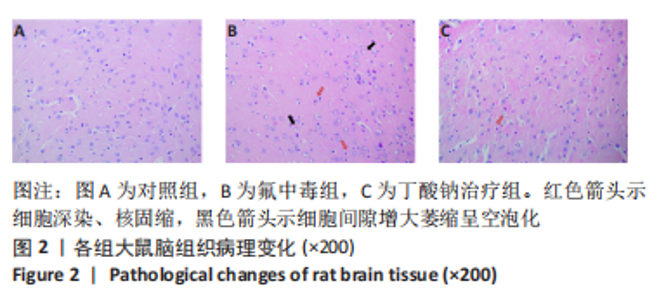
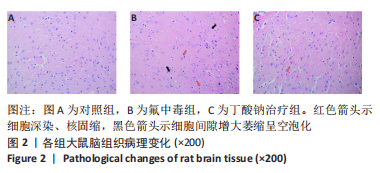
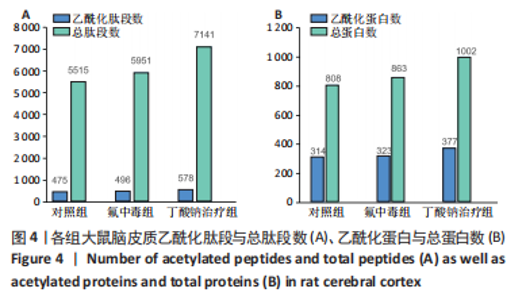
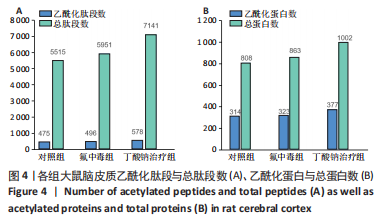
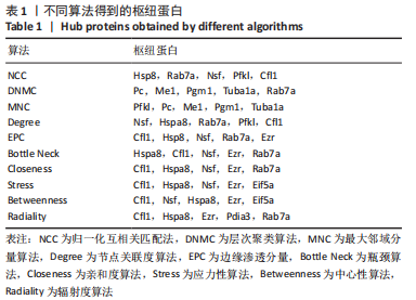
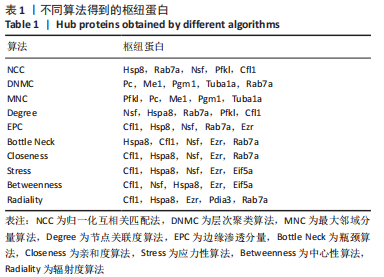
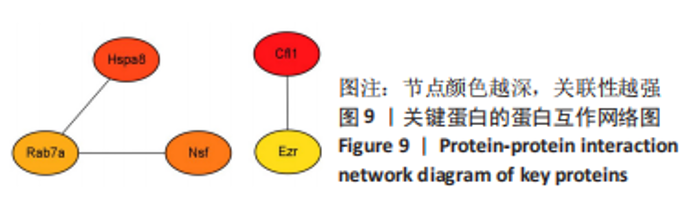
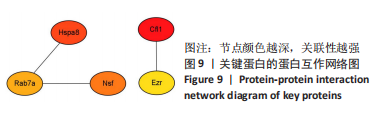
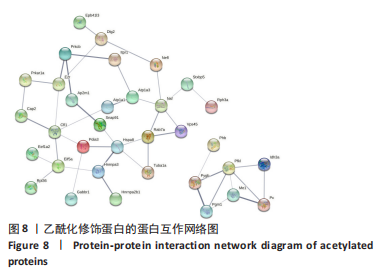
2.8 乙酰化修饰蛋白的互作网络分析 将上述52个蛋白导入STRING数据库绘制互作网络图,最低互动分数设置为0.4,隐藏图中断掉的节点,线条粗细表示数据支持的强弱,获得34个蛋白质节点,边数44的蛋白互作网络图,见图8。将蛋白互作数据导入Cytoscape软件,利用cytohubba插件的不同算法筛选排名前5的枢纽蛋白,见表1,将不同算法的枢纽蛋白出现次数排序,取出现次数最多的前5个为关键蛋白,分别为Hsp8、Rab7a、Nsf、Ezr、Cfl1,见图9。与对照组相比,这些蛋白在氟中毒大鼠脑皮质中并无乙酰化修饰,但经过丁酸钠治疗后,该蛋白出现乙酰化修饰的改变,或为氟神经毒性的致病因素以及丁酸钠潜在的治疗机制,为下一步分子生物学领域的验证奠定基础。"
| [1] SAEED M, MALIK RN, KAMAL A. Fluorosis and cognitive development among children (6-14 years of age) in the endemic areas of the world: a review and critical analysis. Environmental science and pollution research international. 2020;27(3):2566-2579. [2] KERDOUN MA, MEKHLOUFI S, ADJAINE OEK, et al. Fluoride concentrations in drinking water and health risk assessment in the south of Algeria. Regul Toxicol Pharmacol. 2022;128:105086. [3] NING H, LI C, YIN Z, et al. Fluoride exposure decreased neurite formation on cerebral cortical neurons of SD rats in vitro. Environ Sci Pollut Res Int. 2021;28(37):50975-50982. [4] REN C, ZHANG P, YAO XY, et al. The cognitive impairment and risk factors of the older people living in high fluorosis areas: DKK1 need attention. BMC Public Health. 2021;21(1):2237. [5] YANI SI, SEWENG A, MALLONGI A, et al. The influence of fluoride in drinking water on the incidence of fluorosis and intelligence of elementary school students in Palu City. Gac Sanit. 2021;35 Suppl 2: S159-S163. [6] GUPTA R, AMBASTA RK, KUMAR P. Histone deacetylase in neuropathology. Adv Clin Chem. 2021;104:151-231. [7] DAI Y, WEI T, SHEN Z, et al. Classical HDACs in the regulation of neuroinflammation. Neurochem Int. 2021;150:105182. [8] PAO PC, TSAI LH. Histone Deacetylases 1 and 2 in Memory Function. ACS Chem Neurosci. 2022;13(7):848-858. [9] YU Z, HAN J, CHEN H, et al. Oral Supplementation With Butyrate Improves Myocardial Ischemia/Reperfusion Injury via a Gut-Brain Neural Circuit. Front Cardiovasc Med. 2021;8:718674. [10] WANG C, ZHENG D, WENG F, et al. Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes. Psychopharmacology (Berl). 2022;239(1): 215-227. [11] LI X, FAN X, YUAN X, et al. The Role of Butyric Acid in Treatment Response in Drug-Naïve First Episode Schizophrenia. Front Psychiatry. 2021;12:724664. [12] GAO Y, YA B, LI X, et al. Myricitrin ameliorates cognitive deficits in MCAO cerebral stroke rats via histone acetylation-induced alterations of brain-derived neurotrophic factor. Mol Cell Biochem. 2021;476(2): 609-617. [13] JAWORSKA J, ZALEWSKA T, SYPECKA J, et al. Effect of the HDAC Inhibitor, Sodium Butyrate, on Neurogenesis in a Rat Model of Neonatal Hypoxia-Ischemia: Potential Mechanism of Action. Mol Neurobiol. 2019;56(9):6341-6370. [14] 王正东,黄娜,陈婧娴,等.丁酸钠抑制氟中毒可诱导小胶质细胞活化及炎症因子表达增多[J]. 中国组织工程研究,2021,25(7):1075-1080. [15] JIANG Y, LI K, LI X, et al. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem Biol Interact. 2021;341:109452. [16] STILLING RM, VAN DE WOUW M, CLARKE G, et al. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110-132. [17] CODA DM, GRÄFF J. Neurogenetic and Neuroepigenetic Mechanisms in Cognitive Health and Disease. Front Mol Neurosci. 2020;13:205. [18] ZHANG H, ELEFANT F. Exploring the Alzheimer’s disease neuroepigenome: recent advances and future trends. Neural Regen Res. 2022;17(2):325-327. [19] CAMPBELL RR, WOOD MA. How the epigenome integrates information and reshapes the synapse. Nat Rev Neurosci. 2019;20(3):133-147. [20] BURNS AM, GRÄFF J. Cognitive epigenetic priming: leveraging histone acetylation for memory amelioration. Curr Opin Neurobiol. 2021;67:75-84. [21] HECKLAU K, MUELLER S, KOCH SP, et al. The Effects of Selective Inhibition of Histone Deacetylase 1 and 3 in Huntington’s Disease Mice. Front Mol Neurosci. 2021;14:616886. [22] BI N, GU X, FAN A, et al. Bisphenol-A exposure leads to neurotoxicity through upregulating the expression of histone deacetylase 2 in vivo and in vitro. Toxicology. 2022;465:153052. [23] JEONG H, THEN F, MELIA TJ, et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137(1):60-72. [24] KUMAR V, KUNDU S, SINGH A, et al. Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr Neuropharmacol. 2022; 20(1):158-178. [25] KITAHARA M, INOUE T, MANI H, et al. Exercise and pharmacological inhibition of histone deacetylase improves cognitive function accompanied by an increase of gene expressions crucial for neuronal plasticity in the hippocampus. Neurosci Lett. 2021;749:135749. [26] JIANG P, LI G, ZHOU X, et al. Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: Role of GSK-3β/β-catenin pathway. Chemosphere. 2019;214:430-435. [27] MAITY S, FARRELL K, NAVABPOUR S, et al. Epigenetic Mechanisms in Memory and Cognitive Decline Associated with Aging and Alzheimer’s Disease. Int J Mol Sci. 2021;22(22):12280. [28] LEVENSON JM, O’RIORDAN KJ, BROWN KD, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545-40559. [29] GANGULY A, HAN X, DAS U, et al. Hsc70 chaperone activity is required for the cytosolic slow axonal transport of synapsin. J Cell Biol. 2017; 216(7):2059-2074. [30] PARRA LA, BAUST TB, SMITH AD, et al. The Molecular Chaperone Hsc70 Interacts with Tyrosine Hydroxylase to Regulate Enzyme Activity and Synaptic Vesicle Localization. J Biol Chem. 2016;291(34):17510-17522. [31] DOMINGUES R, SANT’ANNA R, DA FONSECA ACC, et al. Extracellular alpha-synuclein: Sensors, receptors, and responses. Neurobiol Dis. 2022;168:105696. [32] DINTER E, SARIDAKI T, NIPPOLD M, et al. Rab7 induces clearance of α-synuclein aggregates. Neurochem. 2016;138(5):758-774. [33] SZEGÖ EM, VAN DEN HAUTE C, HÖFS L, et al. Rab7 reduces α-synuclein toxicity in rats and primary neurons. Exp Neurol. 2022;347:113900. [34] VODICKA P, CHASE K, IULIANO M, et al. Autophagy Activation by Transcription Factor EB (TFEB) in Striatum of HDQ175/Q7 Mice. J Huntingtons Dis. 2016;5(3):249-260. [35] HU K, GAIRE BP, SUBEDI L, et al. Interruption of Endolysosomal Trafficking After Focal Brain Ischemia. Front Mol Neurosci. 2021;14:719100. [36] XI Z, DENG W, WANG L, et al. Association of Alpha-Soluble NSF Attachment Protein with Epileptic Seizure. J Mol Neurosci. 2015;57(3):417-425. [37] CUPERTINO RB, KAPPEL DB, BANDEIRA CE, et al. SNARE complex in developmental psychiatry: neurotransmitter exocytosis and beyond. J Neural Transm (Vienna). 2016;123(8):867-883. [38] MERINO P, DIAZ A, MANRIQUE LG, et al. Urokinase-type plasminogen activator (uPA) promotes ezrin-mediated reorganization of the synaptic cytoskeleton in the ischemic brain. J Biol Chem. 2018;293(24):9234-9247. [39] SONG X, WANG W, WANG H, et al. Acetylation of ezrin regulates membrane-cytoskeleton interaction underlying CCL18-elicited cell migration. J Mol Cell Biol. 2020;12(6):424-437. [40] YU Y, ZENG P, XIONG J, et al. Epigenetic drugs can stimulate metastasis through enhanced expression of the pro-metastatic Ezrin gene. PLoS One. 2010;5(9):e12710. [41] WASIK AA, KOSKELAINEN S, HYVÖNEN ME, et al. Ezrin is down-regulated in diabetic kidney glomeruli and regulates actin reorganization and glucose uptake via GLUT1 in cultured podocytes. Am J Pathol. 2014;184(6):1727-1739. [42] MEDINA C, DE LA FUENTE V, TOM DIECK S, et al. LIMK, Cofilin 1 and actin dynamics involvement in fear memory processing. Neurobiol Learn Mem. 2020;173:107275. [43] SUNGUR A, STEMMLER L, WÖHR M, et al. Impaired Object Recognition but Normal Social Behavior and Ultrasonic Communication in Cofilin1 Mutant Mice. Front Behav Neurosci. 2018;12:25. [44] TSUBOTA T, OKUBO-SUZUKI R, OHASHI Y, et al. Cofilin1 controls transcolumnar plasticity in dendritic spines in adult barrel cortex. PLoS Biol. 2015;13(2):e1002070. |
| [1] | Tao Xin, Xu Yi, Song Zhiwen, Liu Jinbo. Hippo signaling pathway in the regulation of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 619-625. |
| [2] | Zhang Yeting, Fu Yan, Li Xue, Wei Cuilan, Li Chuikun, Yuan Qiongjia. Effects of aerobic exercise on learning, memory, and hippocampal neuromorphology in mice with Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3188-3194. |
| [3] | Tang Yujing, Lan Fengjun, Li Guangdi, Wang Jian, Liu Riguang. Role of calcium ions in the pathogenesis of chronic fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(17): 2745-2753. |
| [4] | . Chinese expert consensus on neurorestorative therapy with cerebrospinal fluid administration (2022) [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(17): 2780-2788. |
| [5] | Liang Weiye, Duan Qinghong. Correlation between femur bone morphogenetic protein 2 expression and bone fluoride content in fluorosis rabbits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(17): 2675-2680. |
| [6] | Sun Honglei, Qi Fengna, Cheng Ruiqing. ICON penetrating resin for treatment of dental fluorosis: 1-year follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(34): 5419-5424. |
| [7] | Wen Xiaoyu, Sun Yuhao, Li Zhuoxian, Xu Lijing, Xia Meng. Network pharmacology and proteomics analysis of Jiawei Xiaoyao San in the treatment of liver cancer complicated with depression in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(32): 5132-5142. |
| [8] | Guan Shihao, Huang Yonghui, Gong Aihua, Cao Xingbing, Sun Haitao, Cai Chuang. Transdifferentiation of rat astrocytes into neurons induced by ectodermal mesenchymal stem cells-derived extracellular vesicles [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4840-4846. |
| [9] | Ding Xue, Jia Ying, Liu Chun, Yang Shirong, Lai Lingyan, Yang Hua, Ding Qi. Displacement and rate changes of orthodontic tooth movement in Sprague-Dawley rats with chronic fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4687-4692. |
| [10] | Yang Jinxin, Wang Kun, Zhao Jing, Chen Peijie, Zhang Tingran, Lu Wenyun, Luo Jiong. Effects of aerobic exercise intervention on synaptic plasticity in Alzheimer’s disease patients [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4216-4223. |
| [11] | Wang Kang, Zhi Xiaodong, Wang Wei. Effect and mechanism of human amniotic epithelial cells on nerve injury repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4046-4051. |
| [12] | Xu Wenshan, Jiang Pingli, Liu Yulu, Ding Yanyi, Yu Yan, Yang Minguang, Liu Weilin, Chen Lidian. Salvianolic acid A effects on hippocampal protein expression in ischemic stroke rats: a tandem mass tag-based proteomic analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3714-3720. |
| [13] | Liu Yulu, Jia Weiwei, Dai Yaling, Xu Wenshan, Ding Yanyi, Liang Shengxiang, Liu Weilin, Chen Lidian. Effects of time-specific AMP-activated protein kinase alpha1/2 gene knockout on hippocampal energy metabolism and synaptic plasticity in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3230-3235. |
| [14] | Shan Zhengming, Tao Shuchun, Hu Chunmei, Zhang Zhiyuan, Ding Yinan, He Mengcheng, Tang Qiusha. Extraction, identification and proteomic analysis of exosomes derived from human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3036-3042. |
| [15] | Hu Yanan, Wang Ping, Du Haitao, Jing Tianyuan, Wang Chengcheng. Mechanism by which antler glue treats avascular necrosis of the femoral head induced by retinoic acid in rats: a serum proteomics study [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2243-2251. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||