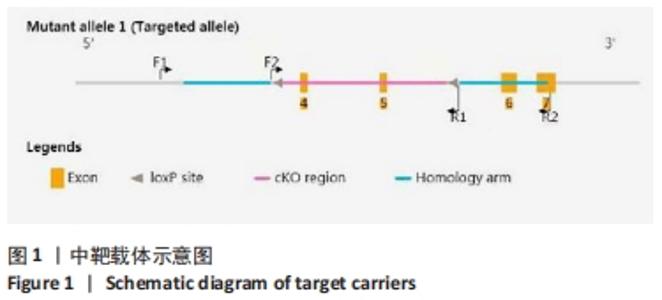
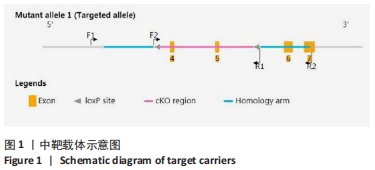
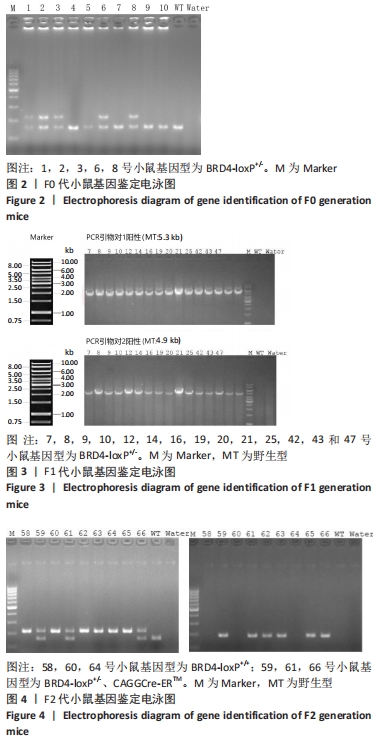
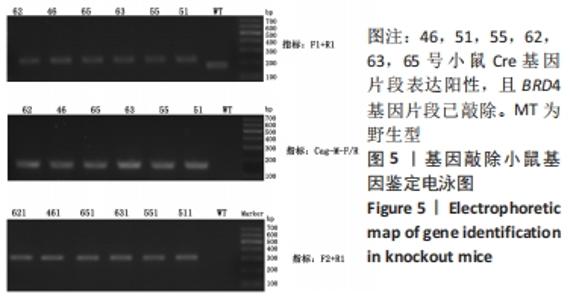
[1] DEY A, NISHIYAMA A, KARPOVA T, et al. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20(23):4899-4909.
[2] TANIGUCHI Y. The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins. Int J Mol Sci. 2016;17(11):1849.
[3] LOVÉN J, HOKE HA, LIN CY, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320-334.
[4] ZUBER J, SHI J, WANG E, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370): 524-528.
[5] DELMORE JE, ISSA GC, LEMIEUX ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904-917.
[6] BAUD’HUIN M, LAMOUREUX F, JACQUES C, et al. Inhibition of BET proteins and epigenetic signaling as a potential treatment for osteoporosis. Bone. 2017;94:10-21.
[7] 任远中. BRD4在类风湿性关节炎骨破坏中的作用机制研究[D]. 青岛:青岛大学,2020.
[8] NAJAFOVA Z, TIRADO-MAGALLANES R, SUBRAMANIAM M, et al. BRD4 localization to lineage-specific enhancers is associated with a distinct transcription factor repertoire. Nucleic Acids Res. 2017;45(1):127-141.
[9] MENG S, ZHANG L, TANG Y, et al. BET Inhibitor JQ1 Blocks Inflammation and Bone Destruction. J Dent Res. 2014;93(7):657-662.
[10] MU J, ZHANG D, TIAN Y, et al. BRD4 inhibition by JQ1 prevents high-fat diet-induced diabetic cardiomyopathy by activating PINK1/Parkin-mediated mitophagy in vivo. J Mol Cell Cardiol. 2020;149:1-14.
[11] LI W, TENG F, LI T, et al. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat Biotechnol. 2013;31(8):684-686.
[12] YANG H, ANG H, SHIVALILA CS, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154(6):1370-1379.
[13] DOUDNA JA, CHARPENTIER E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science (New York, N.Y.). 2014; 346(6213):1258096.
[14] 何晓东, 李江超, 兰天, 等. 乳腺上皮细胞特异性敲除Scrib基因杂合子小鼠的制作与鉴定[J]. 中国比较医学杂志,2013,23(6):28-32.
[15] AUBREY BJ, KELLY GL, KUEH AJ, et al. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep. 2015;10(8):1422-1432.
[16] FLEMR M, BÜHLER M. Single-Step Generation of Conditional Knockout Mouse Embryonic Stem Cells. Cell Rep. 2015;12(4):709-716.
[17] NOIMAN T, KAHANA C. A Simple Combined Use of CRISPR-Cas9 and Cre-LoxP Technologies for Generating Conditional Gene Knockouts in Mammalian Cells. CRISPR J. 2018;1:278-285.
[18] GHOSH K, VAN DUYNE GD. Cre-loxP biochemistry. Methods (San Diego, Calif.). 2002;28(3):374-383.
[19] BOUABE H, OKKENHAUG K. Gene targeting in mice: a review. Methods Mol Biol (Clifton, N.J.). 2013;1064:315-336.
[20] 张杨, 贾林涛, 闫雨冬, 等. Cre-loxP系统及其衍生系统方法学的研究和在神经科学中的应用[J]. 药学学报,2020,55(9):2035-2042.
[21] FEIL R, WAGNER J, METZGER D, et al. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237(3):752-757.
[22] MA Y, YU L, PAN S, et al. CRISPR/Cas9-mediated targeting of the Rosa26 locus produces Cre reporter rat strains for monitoring Cre-loxP-mediated lineage tracing. FEBS J. 2017;284(19):3262-3277.
[23] HUANG, FL, LI F ZHANG WJ, et al. Brd4 participates in epigenetic regulation of the extinction of remote auditory fear memory. Neurobiol Learn Mem. 2021;179:107383.
[24] HENRY SP, JANG CW, DENG JM, et al. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis (New York, N.Y. : 2000). 2009;47(12):805-814.
[25] COUMOUL X, DENG CX. Roles of FGF receptors in mammalian development and congenital diseases. Birth Defects Res C Embryo Today. 2003;69(4):286-304.
[26] FRIEDBERG EC, MEIRA LB. Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage Version 7. DNA Repair (Amst). 2006;5(2):189-209.
[27] LE Y, SAUER B. Conditional gene knockout using cre recombinase. Methods Mol Biol (Clifton, N.J.). 2000;136:477-485.
[28] WEINSTOCK A, GALLEGO-DELGADO J, GOMES C, et al. Tamoxifen activity against Plasmodium in vitro and in mice. Malar J. 2019;18(1): 378-378.
[29] 仝慧慧, 齐浩铭, 王辰, 等. CRISPR/Cas9系统介导Slc6a6基因敲除大鼠的繁殖与鉴定[J]. 中国比较医学杂志,2019,29(5):44-50.
[30] DONATI B, LORENZINI E, CIARROCCHI A. BRD4 and Cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17(1):164.
[31] WU SY, LEE CF, LAI HT, et al. Opposing Functions of BRD4 Isoforms in Breast Cancer. Mol Cell. 2020;78(6):1114-1132.e10.
[32] LIN S, DU L. The therapeutic potential of BRD4 in cardiovascular disease. Hypert Res. 2020;43(10):1006-1014.
[33] WANG H, FU H, ZHU R, et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging. 2020;12(10):9240-9259.
[34] KIM SY, ZHANG X, SCHIATTARELLA GG, et al. Epigenetic Reader BRD4 (Bromodomain-Containing Protein 4) Governs Nucleus-Encoded Mitochondrial Transcriptome to Regulate Cardiac Function. Circulation. 2020;142(24):2356-2370.
[35] DEY A, YANG W, GEGONNE A, et al. BRD4 directs hematopoietic stem cell development and modulates macrophage inflammatory responses. EMBO J. 2019;38(7):e100293.
[36] XIAO Y, LIANG L, HUANG M, et al. Bromodomain and extra-terminal domain bromodomain inhibition prevents synovial inflammation via blocking IkappaB kinase-dependent NF-kappaB activation in rheumatoid fibroblast-like synoviocytes. Rheumatology (Oxford). 2016; 55(1):173-184.
[37] ZHANG QG, QIAN J, ZHU YC. Targeting bromodomain-containing protein 4 (BRD4) benefits rheumatoid arthritis. Immunol Lett. 2015;166(2): 103-108.
[38] 许颖华, 姜龙, 史春. 慢性根尖周炎模型大鼠表观遗传分子:溴结构域蛋白4的表达变化[J]. 中国组织工程研究,2022,26(14):2167-2171.
[39] REN Y, ZHANG Y, WANG Z, et al. Role of Brd4 in the production of inflammatory cytokines in mouse macrophages treated with titanium particles. Can J Physiol Pharmacol. 2019;97(11):1028-1034.
[40] GUO NH, ZHENG JF, ZI FM, et al. I-BET151 suppresses osteoclast formation and inflammatory cytokines secretion by targetting BRD4 in multiple myeloma. Biosci Rep. 2019;39(5):BSR20181245.
|