Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (20): 3184-3189.doi: 10.12307/2022.618
Previous Articles Next Articles
Effects of silent information regulator 1 on chronic heart failure in a postmenopausal mouse model and its mechanism
Zhang Haiyang1, Bi Shengli2, Feng Jingru1, Li Fan1, Wang Jing1, Zhao Na1, Li Xinjun1
- 1Department of Cardiology, 2Department of Gynecology, the Second Affiliated Hospital of Hebei North University, Zhangjiakou 075100, Hebei Province, China
-
Received:2021-08-02Accepted:2021-09-03Online:2022-07-18Published:2022-01-19 -
Contact:Bi Shengli, Chief physician, Department of Gynecology, the Second Affiliated Hospital of Hebei North University, Zhangjiakou 075100, Hebei Province, China -
About author:Zhang Haiyang, Associate chief physician, Department of Cardiology, the Second Affiliated Hospital of Hebei North University, Zhangjiakou 075100, Hebei Province, China -
Supported by:2019 Medical Science Research Project of Hebei Provincial Health Commission, No. 20190867 (to ZHY)
CLC Number:
Cite this article
Zhang Haiyang, Bi Shengli, Feng Jingru, Li Fan, Wang Jing, Zhao Na, Li Xinjun. Effects of silent information regulator 1 on chronic heart failure in a postmenopausal mouse model and its mechanism[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3184-3189.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

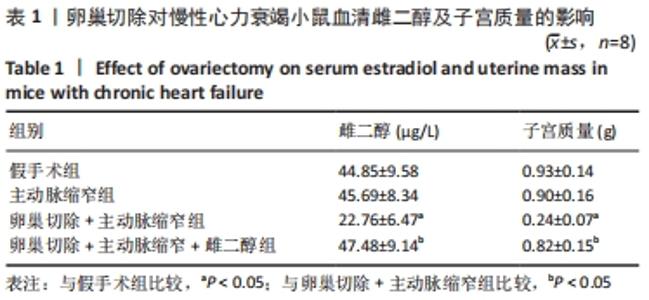
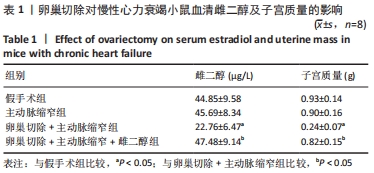
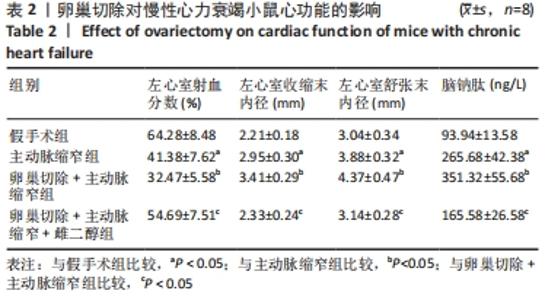
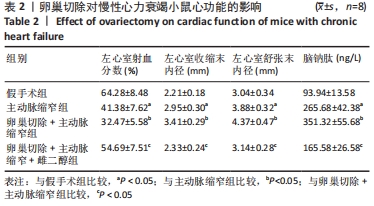
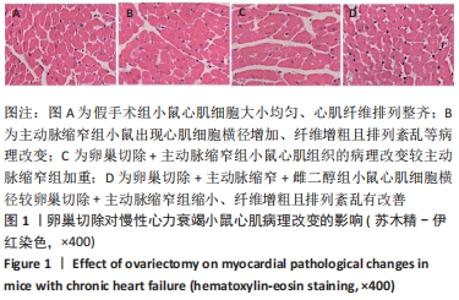
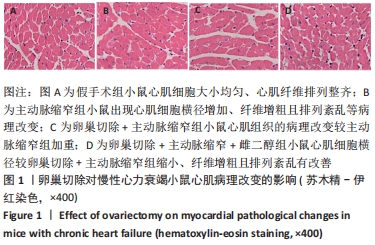
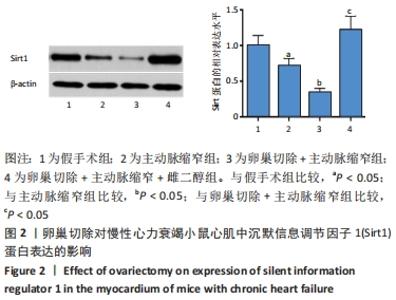
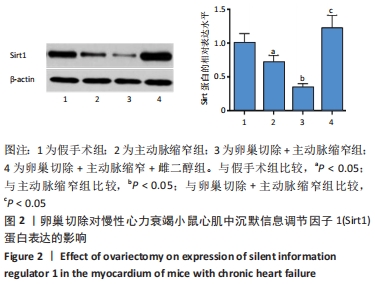
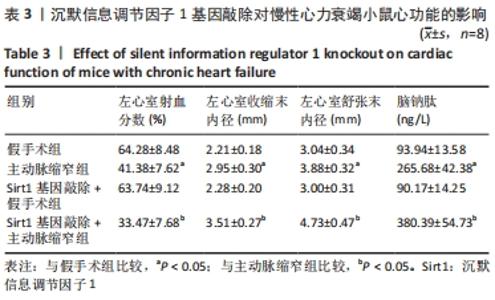
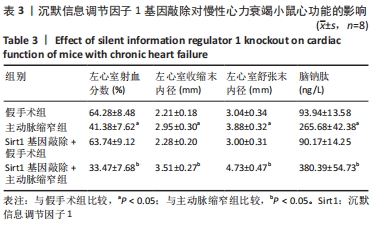
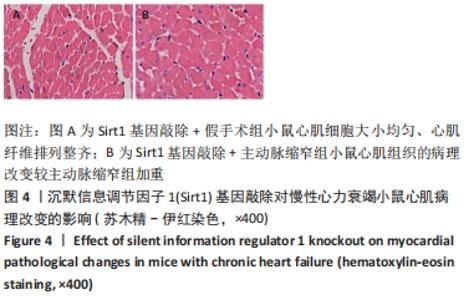
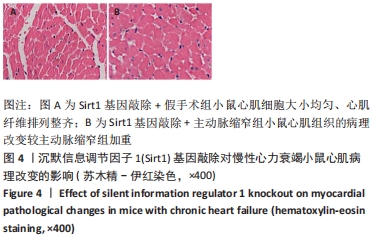
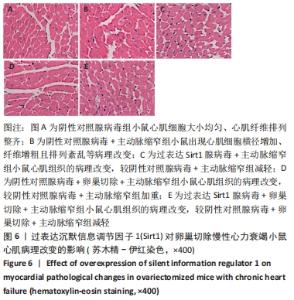
2.3 第二部分实验结果 2.3.1 Sirt1基因敲除对慢性心力衰竭小鼠心功能的影响 Sirt1基因敲除+假手术组及Sirt1基因敲除+主动脉缩窄组小鼠心肌中均不表达Sirt1蛋白,见图3。与假手术组比较,主动脉缩窄组小鼠的左心室射血分数水平显著降低,左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平均显著增加(P < 0.05),Sirt1基因敲除+假手术组左心室射血分数水平、左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平无显著变化(P > 0.05);与主动脉缩窄组比较,Sirt1基因敲除+主动脉缩窄组小鼠的左心室射血分数水平显著降低,左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平均显著增加(P < 0.05),见表3。"

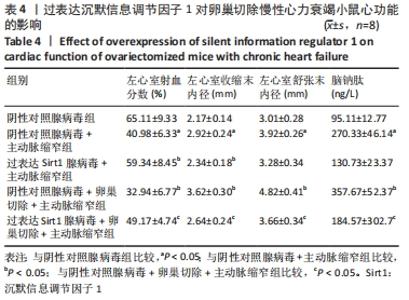
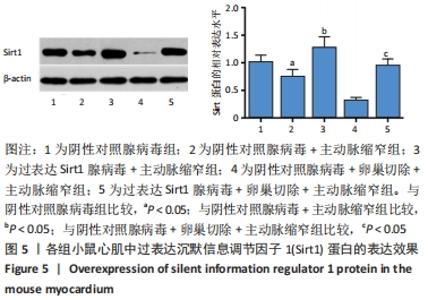
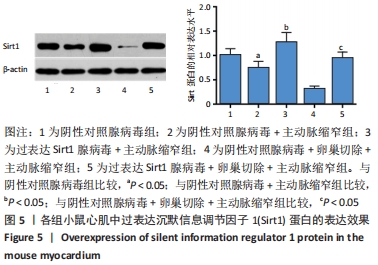
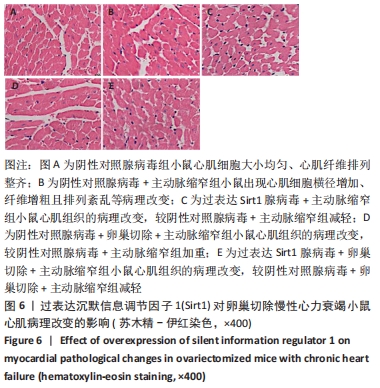
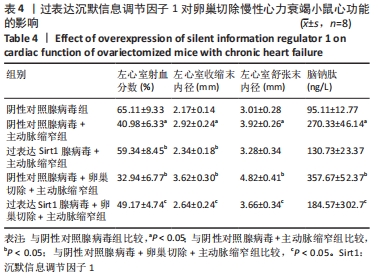
2.4.2 过表达Sirt1对卵巢切除慢性心力衰竭小鼠心功能的影响 与阴性对照腺病毒组比较,阴性对照腺病毒+主动脉缩窄组小鼠的左心室射血分数水平显著降低,左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平均显著增加(P < 0.05);与阴性对照腺病毒+主动脉缩窄组比较,过表达Sirt1腺病毒+主动脉缩窄组小鼠的左心室射血分数水平显著增加,左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平均显著降低(P < 0.05),阴性对照腺病毒+卵巢切除+主动脉缩窄组小鼠的左心室射血分数水平显著降低,左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平均显著增加 (P < 0.05);与阴性对照腺病毒+卵巢切除+主动脉缩窄组比较,过表达Sirt1腺病毒+卵巢切除+主动脉缩窄组小鼠的左心室射血分数水平显著增加,左心室收缩末内径、左心室舒张末内径值及血清脑钠肽水平均显著降低(P < 0.05),见表4。"
| [1] FREANEY PM, KHAN SS, LLOYD-JONES DM, et al. The Role of Sex-Specific Risk Factors in the Risk Assessment of Atherosclerotic Cardiovascular Disease for Primary Prevention in Women. Curr Atheroscler Rep. 2020;22(9):46. [2] ZHU D, CHUNG HF, DOBSON AJ, et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies. Hum Reprod. 2020;35(8):1933-1943. [3] 汤展, 马懿, 钟元林, 等. 绝经后冠心病患者血浆雌二醇与心室重构及NT-proBNP的相关性[J]. 中国老年学杂志,2015,35(9):2381-2383. [4] 拓步雄, 徐杰, 邹倩, 等. 慢性心力衰竭血清Hcy、MIP-1α、SIRT1水平检测的价值研究[J]. 解放军医药杂志,2020,32(10):36-39,44. [5] SASAKI Y, IKEDA Y, MIYAUCHI T, et al. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J Atheroscler Thromb. 2020;27(1):47-59. [6] GUO JM, SHU H, WANG L, et al. SIRT1-dependent AMPK pathway in the protection of estrogen against ischemic brain injury. CNS Neurosci Ther. 2017;23(4):360-369. [7] 雷迁, 魏新川, 徐金东, 等. 超声评价微创横向主动脉缩窄小鼠模型心脏重构变化[J]. 西部医学,2019,31(4):503-507. [8] CHEN H, LIU S, ZHAO C, et al. Cardiac contractility modulation improves left ventricular systolic function partially via miR-25 mediated SERCA2A expression in rabbit trans aortic stenosis heart failure model. J Thorac Dis. 2018;10(6):3899-3908. [9] AHMAD F, SINGH AP, TOMAR D, et al. Cardiomyocyte-GSK-3alpha promotes mPTP opening and heart failure in mice with chronic pressure overload. J Mol Cell Cardiol. 2019;130:65-75. [10] LOPES ACR, ZAVAN B, CORRÊA YJC, et al. Impact of obesity and ovariectomy on respiratory function in female mice. Respir Physiol Neurobiol. 2021; 17(294):103775. [11] Wang X, Lu L, Tan Y, et al. GPR 30 reduces myocardial infarct area and fibrosis in female ovariectomized mice by activating the PI3K/AKT pathway. Life Sci. 2019;1(226):22-32. [12] CAMARGO TF, ZANESCO AM, PACHER KAS, et al. Physiological profile regulation during weight gain and loss by ovariectomized females: importance of SIRT1 and SIRT4. Am J Physiol Endocrinol Metab. 2020; 319(4):E769-E778. [13] JIANG Y, LUO W, WANG B, et al. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020;1(246): 117422. [14] NIE Q, ZHANG J, HE B, et al. A novel mechanism of protection against isoproterenol-induced cardiac inflammation via regulation of the SIRT1/NRF2 signaling pathway with a natural SIRT1 agonist. Eur J Pharmacol. 2020;5(886):173398. [15] HWANG DK, CHANG YL, LIN TC, et al. Changes in the Systemic Expression of Sirtuin-1 and Oxidative Stress after Intravitreal Anti-Vascular Endothelial Growth Factor in Patients with Retinal Vein Occlusion. Biomolecules. 2020; 10(10):1414. [16] LI B, LI M, LI X, et al. Sirt1-inducible deacetylation of p21 promotes cardiomyocyte proliferation. Aging (Albany NY). 2019;11(24):12546-12567. [17] LIN B, ZHAO H,LI L,et al. Sirt1 improves heart failure through modulating the NF-kappaB p65/microRNA-155/BNDF signaling cascade. Aging (Albany NY). 2020;18:12. [18] VÁZQUEZ-GARZA E, BERNAL-RAMÍREZ J, JERJES-SÁNCHEZ C, et al. Resveratrol Prevents Right Ventricle Remodeling and Dysfunction in Monocrotaline-Induced Pulmonary Arterial Hypertension with a Limited Improvement in the Lung Vasculature. Oxid Med Cell Longev. 2020;3(2020):1841527. |
| [1] | Li Wei, Zhu Hanmin, Wang Xin, Gao Xue, Cui Jing, Liu Yuxin, Huang Shuming. Effect of Zuogui Wan on bone morphogenetic protein 2 signaling pathway in ovariectomized osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1173-1179. |
| [2] | Jing Jin, Zhao Shandi, Chen Long, Peng Shuanglin, Tang Hui, Guo Daijin, Zeng Xinyi, Xiao Jingang. Repair of calvarial defects in osteoporotic mice by adipose-derived stem cells combined with biphasic calcium phosphate ceramic scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 90-95. |
| [3] | Zuo Bin, Xia Xiaofeng, Che Biao, Zou Kai, Tang Jiaguo. Polydatin effects on apoptosis of nucleus pulposus cells and SIRT1/mTOR pathway in mice with lumbar disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(35): 5619-5625. |
| [4] | Li Shengqiang, Xie Bingying, Chen Juan, Xie Lihua, Huang Jingwen, Ge Jirong. Interaction proteomics of long noncoding RNA uc431+ gene in postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(11): 1641-1646. |
| [5] | Zhao Jing. Effects of Tai Chi Chuan on the changes of bone mineral density of perimenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(2): 176-180. |
| [6] | Wu Yuhang, Zheng Liqin, Zhang Biao, Li Fan, Chen Xinmin, Li Musheng, Zheng Yongze, Lin Ziling. Compression fracture simulation of osteoporotic trabecular bone in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(15): 2387-2392. |
| [7] | Zhang Lei1, Zeng Gao-feng1, Zong Shao-hui2, Wu Ping-ping1, He Ji-chen2, Wu Yun-le2, Yan Fang-na1, Qin Zhong-xi2, Huang Jian-hua2. Molecular mechanism of polygonatum sibiricum polysaccharide in the prevention and treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(4): 493-498. |
| [8] | Huang Kui1, Luo Dao-wen1, Wang Lei1, Luo Shi-hong1, Yao Zhi-hao1, Li Yong1, Xiao Jin-gang1, 2. Establishing a rat model of osteoporosis with critical-size calvarial defects [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(8): 1236-1240. |
| [9] | Luo Dao-wen1, Huang Kui1, Wang Lei1, Luo Shi-hong1, Rao Peng-cheng1, Xiao Jin-gang1, 2. Establishment of a C57 mouse model of osteoporosis with calvarial critical-size defects [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(36): 5793-5798. |
| [10] | Jia Zhi, Zhang Feng-wei, Qi Xing-ying, Yan Xiao, Hua Ye, Zhao Meng-ming, Liu Da-yong. Effect of estrogen deficiency on the expression levels of nuclear factor-kappa B and interleukin-17 in periodontal tissues [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(24): 3796-3802. |
| [11] | Xue Ai-cun, Wang Da-shou, Zhong Jie, Deng Chun-tao, Yu Bin, Shi Hua-xin, Mu Jiang-ping. Effect of lumbosacral plexus block on the serum levels of hormones in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(16): 2540-2545. |
| [12] | Mu Shu-lin, Zhang Liu, Tian Fa-ming, Mu Shu-min, Sun Li-xia, Chu Li-ming. Comparison of the effects of parathyroid hormone (1-34), alendronate sodium and simvastatin on osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(46): 6854-6860. |
| [13] | Su Fan, Lin Jing-xia, Wu Li-qiu, Cai Dong-mei, Zhuang Ze. Effects of alendronate sodium versus hormone replacement therapy for osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(46): 6893-6898. |
| [14] | Liang Yao-zhong1, Chen Shu2, Yang Yu-hao1, Lan Chun-hai1, Zhang Guo-wei1, Ji Zhi-sheng1, . Atorvastatin promotes implant osseointegration via the activation of Wnt/β-catenin signal pathway in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(20): 2940-2948. |
| [15] | Qi Bing, Dai Wei-qun, You Jian-yu, Li Xiao-li. Femoral compression variation in osteoporosis rats after intervention with different drugs [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(42): 6770-6775. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||