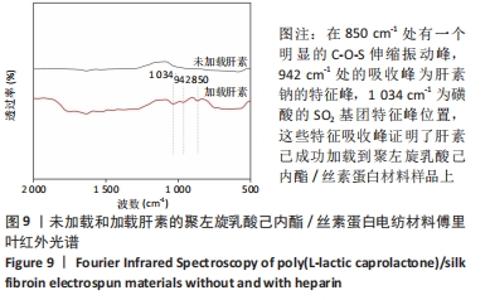
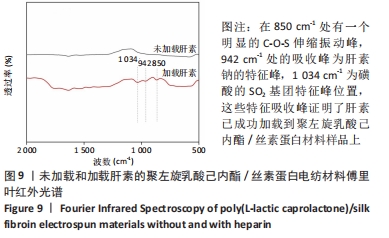
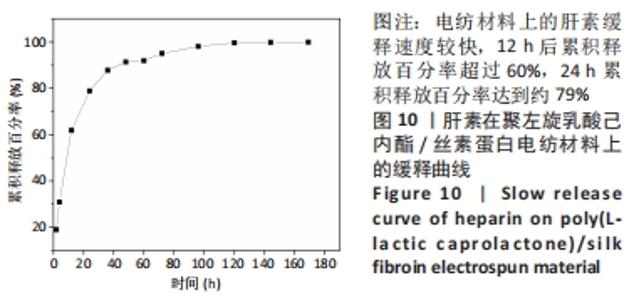
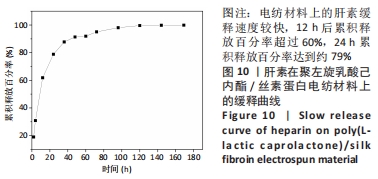
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (22): 3505-3513.doi: 10.12307/2022.278
Previous Articles Next Articles
Cell co-culture and in vivo biocompatibility of poly(L-lactic caprolactone)/silk fibroin small-diameter artificial blood vessels
Liu Yue1, Jiang Ziyi1, Li Jingjing2,3, Meng Kai1, Zhao Huijing1
- 1College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, Jiangsu Province, China; 2Institute for Cardiovascular Science, Soochow University, Suzhou 215021, Jiangsu Province, China; 3Department of Cardiovascular Surgery, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2021-05-14Revised:2021-07-22Accepted:2021-08-05Online:2022-08-08Published:2022-01-12 -
Contact:Zhao Huijing, MD, Associate professor, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, Jiangsu Province, China -
About author:Liu Yue, Master, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, Jiangsu Province, China -
Supported by:Natural Science Foundation for Key Program of Jiangsu Higher Education Institutions, No. 19KJA610004 (to ZHJ); Science and Technology Guidance Project of China Textile Industry Federation, No. 2019021 (to ZHJ)
CLC Number:
Cite this article
Liu Yue, Jiang Ziyi, Li Jingjing, Meng Kai, Zhao Huijing. Cell co-culture and in vivo biocompatibility of poly(L-lactic caprolactone)/silk fibroin small-diameter artificial blood vessels[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3505-3513.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
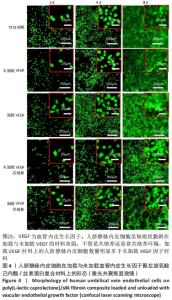
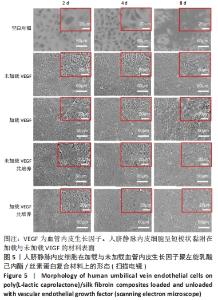
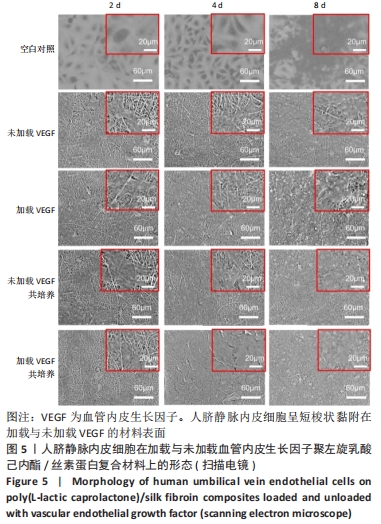
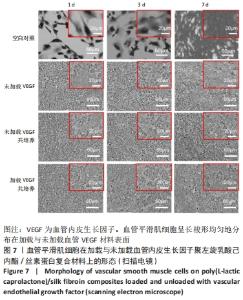
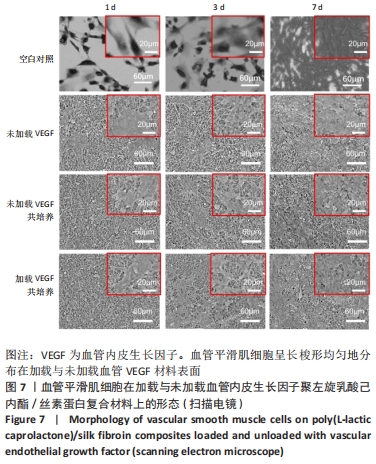
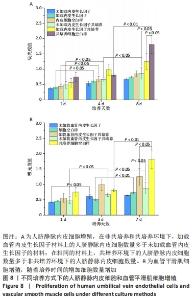
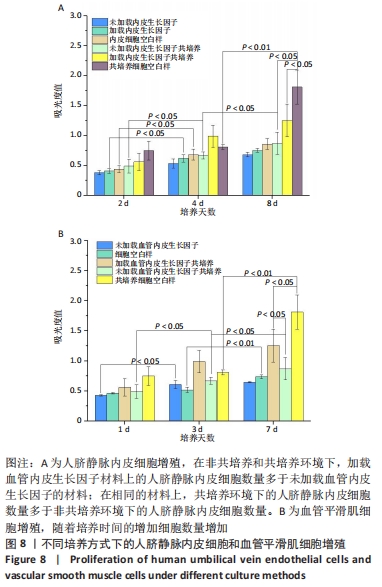
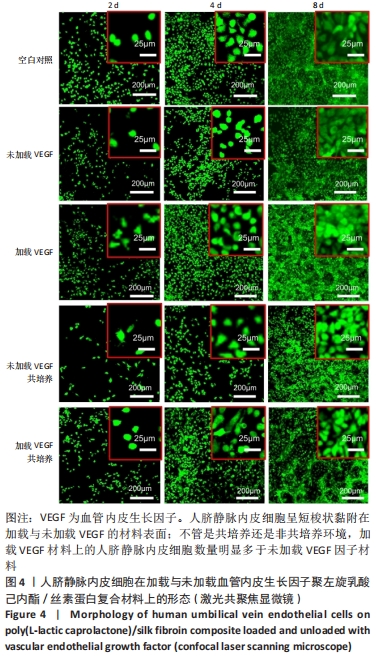
2.1 不同培养方式下的两种细胞形态 2.1.1 人脐静脉内皮细胞 由图4,5的激光共聚焦显微镜和扫描电镜图可以看出,人脐静脉内皮细胞呈短梭状黏附在材料表面。虽然人脐静脉内皮细胞在聚左旋乳酸己内酯/丝素蛋白复合材料上的细胞数量都低于空白对照组,但聚左旋乳酸己内酯/丝素蛋白复合材料上的人脐静脉内皮细胞数量随着培养天数的增加而增加,在第8天时细胞与细胞连接成片状。不管是共培养还是非共培养环境,加载血管内皮生长因子材料上人脐静脉内皮细胞数量明显多于未加载血管内皮生长因子材料。加载血管内皮生长因子材料上的人脐静脉内皮细胞在第8天时基本覆盖材料表面,甚至部分细胞堆叠在一起。在相同的材料上,共培养环境下的人脐静脉内皮细胞数量多于非共培养环境下的人脐静脉内皮细胞数量。"
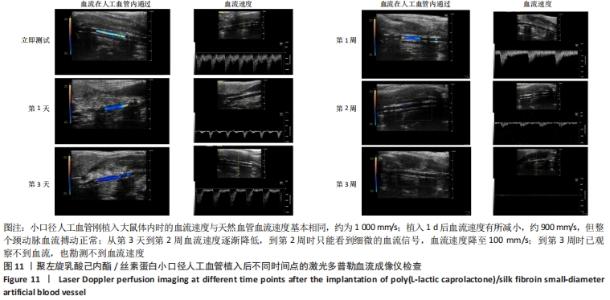
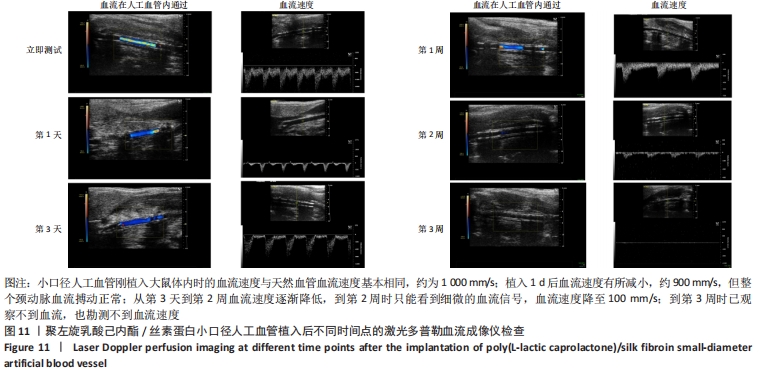
2.4 人工血管动物体内植入实验结果 2.4.1 通畅性检查 手术完成之时与术后第1,3天及1,2,3周,利用激光多普勒血流成像仪检查聚左旋乳酸己内酯/丝素蛋白小口径人工血管的通畅性,见图11。大鼠天然血管的血流速度大约为1 000 mm/s,聚左旋乳酸己内酯/丝素蛋白小口径人工血管刚植入大鼠体内时的血流速度与天然血管血流速度基本相同;植入1 d后血流速度有所减小,约900 mm/s,但整个颈动脉血流搏动正常;从第3天到第2周血流速度逐渐降低,到第2周时只能看到细微的血流信号,血流速度降至100 mm/s;到第3周时,从多普勒血流成像仪中已观察不到血流,也勘测不到血流速度,但解剖取样时发现,当把远心端血管剪开时仍然有近心端的血流从小口径人工血管流出。"
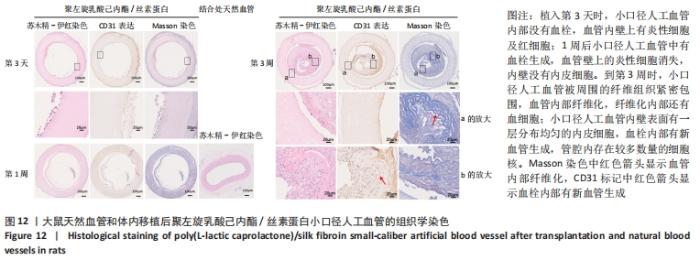
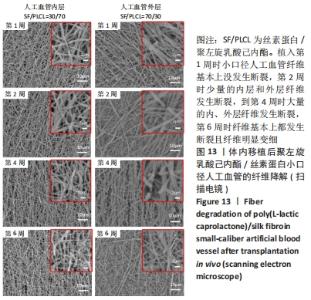
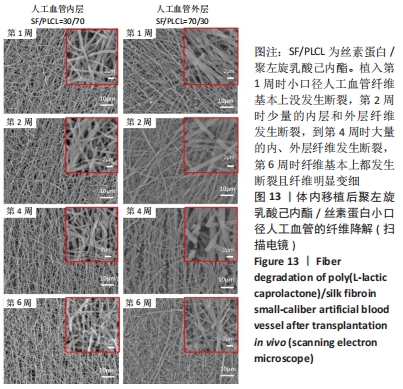
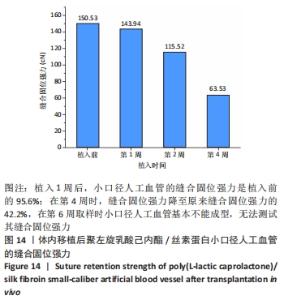
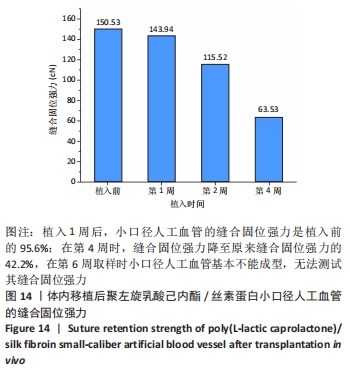
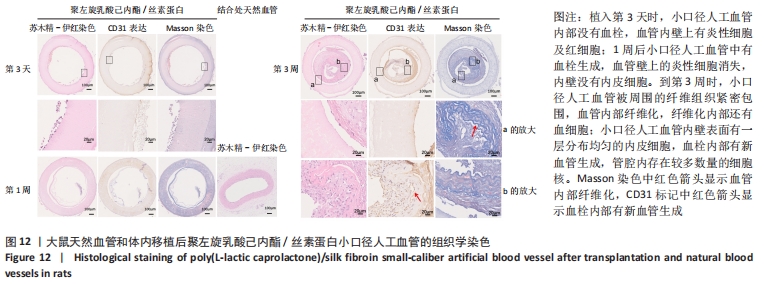
2.4.2 组织学分析 图12为聚左旋乳酸己内酯/丝素蛋白小口径人工血管和大鼠颈动脉天然血管的组织分析图。从苏木精-伊红染色图中可以看出,大鼠天然血管上的内皮细胞核呈蓝色,内弹性膜呈波浪形红色等结构特点。在CD31免疫组化染色中内皮细胞呈棕黄色,在Masson染色中胶原纤维呈蓝色[30]。植入第3天时,小口径人工血管内部没有血栓,通过苏木精-伊红染色与Masson染色对比发现,聚左旋乳酸己内酯/丝素蛋白小口径人工血管内壁上有炎性细胞及红细胞;1周后聚左旋乳酸己内酯/丝素蛋白小口径人工血管中有血栓生成,血管壁上的炎性细胞消失,CD31免疫组化染色结果显示小口径人工血管内壁没有内皮细胞。到第3周时,聚左旋乳酸己内酯/丝素蛋白小口径人工血管被周围的纤维组织紧密包围,根据Masson染色发现血管内部纤维化,纤维化内部还有血细胞,说明管内部分为血栓;从苏木精-伊红染色和CD31免疫组化染色放大图可以看出,聚左旋乳酸己内酯/丝素蛋白小口径人工血管内壁表面有一层分布均匀的内皮细胞;CD31免疫组化染色显示在血栓内部有新血管生成,管腔内存在较多数量的细胞核,提示内膜增生的可能性。"
| [1] YANG L, LI X, WU Y, et al.Preparation of PU/Fibrin Vascular Scaffold with Good Biomechanical Properties and Evaluation of Its Performance in vitro and in vivo. 2020;15:8697-8715. [2] LI J, LONG Y, YANG F, et al. Multifunctional Artificial Artery from Direct 3D Printing with Built‐In Ferroelectricity and Tissue‐Matching Modulus for Real‐Time Sensing and Occlusion Monitoring. Adv Funct Mater. 2020;30(39):2002868. [3] WOUK J, DEKKER RFH, QUEIROZ EAIF, et al. β-Glucans as a panacea for a healthy heart? Their roles in preventing and treating cardiovascular diseases. Int J Biol Macromol. 2021;177:176-203. [4] EL-GHAZALI S, KHATRI M, HUSSAIN N, et al. Characterization and biocompatibility evaluation of artificial blood vessels prepared from pristine poly (Ethylene-glycol-co-1,4-cyclohexane dimethylene-co-isosorbide terephthalate), poly (1, 4 cyclohexane di-methylene-co-isosorbide terephthalate) nanofibers and their blended composition. Mater Today Commun. 2021;26:102113. [5] GAO A, HANG R, LI W, et al. Linker-free covalent immobilization of heparin, SDF-1α, and CD47 on PTFE surface for antithrombogenicity, endothelialization and anti-inflammation. Biomaterials. 2017;140:201-211. [6] GEELHOED WJ, van der BOGT KEA, ROTHUIZEN TC, et al. A novel method for engineering autologous non-thrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting. Biomaterials. 2020;229:119577. [7] KABIRIAN F, DITKOWSKI B, ZAMANIAN A, et al. Controlled NO-Release from 3D-Printed Small-Diameter Vascular Grafts Prevents Platelet Activation and Bacterial Infectivity. ACS Biomater Sci Eng. 2019;5(5):2284-2296. [8] SHI C, TINGTING W, LI J, et al. Comprehensive Landscape of Heparin Therapy for COVID-19. Carbohydr Polym. 2021;254:117232. [9] MANABE K, NARA H. Construction of stable biological albumin/heparin multilayers for elastic coatings on hydrophobic antithrombogenic artificial blood vessels. Tribol Int. 2021;156:106843. [10] KUANG H, WANG Y, SHI Y, et al. Construction and performance evaluation of Hep/silk-PLCL composite nanofiber small-caliber artificial blood vessel graft. Biomaterials. 2020;259:120288. [11] EMECHEBE GA, OBIWELUOZOR FO, JEONG IS, et al. Merging 3D printing with electrospun biodegradable small-caliber vascular grafts immobilized with VEGF. Nanomed-Nano Technol. 2020;30:102306. [12] APTE RS, CHEN DS, FERRARA N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell (Cambridge). 2019;176(6):1248-1264. [13] AYDOGDU MO, CHOU J, ALTUN E, et al. Production of the biomimetic small diameter blood vessels for cardiovascular tissue engineering. Int J Polym Mater. 2019;68(5):243-255. [14] TONDREAU MY, LATERREUR V, GAUVIN R, et al. Mechanical properties of endothelialized fibroblast-derived vascular scaffolds stimulated in a bioreactor. Acta Biomater. 2015;18:176-185. [15] TIWARI A, CHENG KS, SALACINSKI H, et al. Improving the patency of vascular bypass grafts: The role of suture materials and surgical techniques on reducing anastomotic compliance mismatch. Eur J Vasc Endovasc Surg. 2003;25(4):287-295. [16] GREENWALD SE, BERRY CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000;190(3):292-299. [17] GAO J, CRAPO P, NEREM R, et al. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J Biomed Mater Res A. 2008;85A(4):1120-1128. [18] ZHANG Y, LIU Y, JIANG Z, et al. Poly(glyceryl sebacate)/silk fibroin small-diameter artificial blood vessels with good elasticity and compliance. Smart Mater Med. 2021;2:74-86. [19] DIMOPOULOS A, MARKATOS DN, MITROPOULOU A, et al. A novel polymeric fibrous microstructured biodegradable small-caliber tubular scaffold for cardiovascular tissue engineerin. J Mater Sci Mater Med. 2021;32(2):21. [20] 关颖,关国平,林婧,等.小口径人工血管顺应性的影响因素和改善方法[J].材料导报,2014,28(19):125-129. [21] ZHENG M, GUO J, LI Q, et al. Syntheses and characterization of anti-thrombotic and anti-oxidative Gastrodin-modified polyurethane for vascular tissue engineering. Bioact Mater. 2021;6(2):404-419. [22] LE AN, TRAN NM, PHAN TB, et al. Poloxamer additive as luminal surface modification to modulate wettability and bioactivities of small-diameter polyurethane/polycaprolactone electrospun hollow tube for vascular prosthesis applications. Mater Today Commun. 2021;26:101771. [23] YANG L, LI X, WU Y, et al. Preparation of PU/Fibrin Vascular Scaffold with Good Biomechanical Properties and Evaluation of Its Performance in vitro and in vivo . 2020;15:8697-8715.[24] YANG X, WEI J, LEI D, et al. Appropriate density of PCL nano-fiber sheath promoted muscular remodeling of PGS/PCL grafts in arterial circulation. Biomaterials. 2016;88:34-47. [25] JIN D, WU S, KUANG H, et al. Preliminary application of a cell-free mono-layered vascular scaffold in a rabbit model. Mater Design. 2021; 198:109301. [26] MATSUZAKI Y, IWAKI R, REINHARDT JW, et al. The effect of pore diameter on neo-tissue formation in electrospun biodegradable tissue-engineered arterial grafts in a large animal model. Acta Biomater. 2020; 115:176-184. [27] QIN K, WANG F, SIMPSON RML, et al. Hyaluronan promotes the regeneration of vascular smooth muscle with potent contractile function in rapidly biodegradable vascular grafts. Biomaterials. 2020; 257:120226. [28] KHODADOUST M, MOHEBBI-KALHORI D, JIROFTI N. Fabrication and Characterization of Electrospun Bi-Hybrid PU/PET Scaffolds for Small-Diameter Vascular Grafts Applications. Cardiovasc Eng Techn. 2018;9(1):73-83. [29] LI X, ZHAO H. Mechanical and degradation properties of small-diameter vascular grafts in anin vitro biomimetic environment. J Biomater Appl. 2019;33(8):1017-1034. [30] HONG JH, KIM DH, RHYU IJ, et al. A simple morphometric analysis method for dermal microstructure using color thresholding and moments. Skin Res Technol. 2020;26(1):132-136. [31] HENSHALL TL, KELLER A, HE L, et al. Notch3 Is Necessary for Blood Vessel Integrity in the Central Nervous System. Arterioscler Thromb Vasc Biol. 2015;35(2):409-420. [32] KHAN AUR, HUANG K, JINZHONG Z, et al. PLCL/Silk fibroin based antibacterial nano wound dressing encapsulating oregano essential oil: Fabrication, characterization and biological evaluation. Colloids Surf B Biointerfaces. 2020;196:111352. [33] GAO H, XU S, LI J, et al. Tirofiban Promotes the Proliferation of Human Umbilical Vein Endothelial Cells In Vitro Via Enhanced Vascular Endothelial Growth Factor Expression. Transpl P. 2020;52(1):419-422. [34] SOLOGASHVILI T, SAAT SA, TILLE J, et al. Effect of implantation site on outcome of tissue-engineered vascular grafts. Eur J Pharm and Biopharm. 2019;139:272-278. [35] 华楠.生物降解材料的体内降解机理[J].国外医学(生物医学工程分册),2004,(3):181-184. |
| [1] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [2] | Jiang Chaorui, Xu Yan, Xiong Ying, Zhang Xujing. Preparation and properties of graphene oxide/silk fibroin/rifampicin drug-loading microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3467-3473. |
| [3] | Meng Lulu, Liu Hao, Liu Han, Zhang Jun, Li Ruixin, Gao Lilan. Mechanical properties of silk fibroin/type I collagen/hydroxyapatite scaffolds based on low-temperature 3D printing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3550-3555. |
| [4] | Guo Xiaopeng, Liu Yingsong, Shang Hui. Silk fibroin/nano hydroxyapatite composite combined with icariin can promote the proliferation and differentiation of bone marrow mesenchymal stem cells into nucleus pulposus like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3528-3534. |
| [5] | Yang Xinghua, Zhang Jing, Chen Daiyun, Xiong Shijiang. Selection of conditions for fabricated porous scaffolds in bone tissue engineering by silk fibroin protein [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2545-2550. |
| [6] | An Tiantian, Xu Yan, Chen Huiming, Zhang Xujing, Tan Hao. Preparation and performance of rifampicin-silk fibroin microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1515-1521. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Liu Xiaoyuan, Li Lei, Zhang Kai, Li Jun, Han Xiangzhen He Huiyu. Osteogenesis using bone marrow mesenchymal stem cell sheets combined with three-dimensional printed Cervus elaphus antler powder/silk fibroin/polyvinyl alcohol scaffold in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5420-5426. |
| [9] | Chen Zhenyu, Zhang Xiaoning, Luo Yuxin, Liang Jianwei, Yan Chi. Evaluation of silk fibroin/curcumin composite film for promoting wound healing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2554-2561. |
| [10] | Xu Changkui, Pu Xiaobing, Lu Yao, Chen Jiarong, Pan Lei. Safety and antibacterial properties of gentamicin-loaded silk fibroin in meniscus repair [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1545-1549. |
| [11] | Liu Xiaoyin, , Zhong Lin, Zheng Bo, Wei Pan, Dai Chen, Hu Liangcong, Wang Tiantian, Liang Xiaolong, Zhang Sai, Wang Xiaoli. Diffusion tensor imaging predicting locomotor function recovery with 3D printing scaffold after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4547-4554. |
| [12] | Zhang Xiaoyun, Chen Yueping, Song Shilei, Zhang Chi, Zhuo Yinghong, Yang Nan, Zhan Huasong, Xu Canhong. Good cell compatibility and permeability of silk fibroin/chitosan composite scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2544-2550. |
| [13] | She Rongfeng, Zhang Yi, Chen Long, Wang Yuanzheng, Zhang Bin, Huang Qixiang. Biocompatibility of tissue engineered cartilage constructed in vivo by silk fibroin-chitosan scaffold carrying bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(1): 27-32. |
| [14] | Chen Lei, Ge Weiming, Lü Linwei, Lei Ming, Teresa Zielińska, Xing Enhong. Mechanical properties of artificial cartilage scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(30): 4763-4768. |
| [15] | Sun Kai, Li Ruixin, Li Hao, Li Dong, Li Hui. Finite element analysis and demonstration of scaffold material stacking [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(30): 4787-4792. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||