中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (36): 7827-7838.doi: 10.12307/2025.502
• 干细胞综述 stem cell review • 上一篇 下一篇
负载miRNA间充质干细胞源外泌体改善脊髓损伤的作用机制
郭 佳1,2,任亚锋2,李 冰2,黄 靖1,尚文雅1,杨溢珂1,刘慧瑶1
- 1河南中医药大学康复医学院,河南省郑州市 450046;2河南中医药大学第一附属医院,河南省郑州市 450000
-
收稿日期:2024-04-08接受日期:2024-04-24出版日期:2025-12-28发布日期:2025-03-14 -
通讯作者:任亚锋,博士,主任医师,硕士生导师,河南中医药大学第一附属医院,河南省郑州市 450000 -
作者简介:郭佳,女,2001年生,山西省吕梁市人,汉族,河南中医药大学在读硕士,主要从事脊髓损伤康复治疗方面的研究。 -
基金资助:河南省中医药科学研究专项课题 (2021JDZY022,2022JDZY015),项目负责人:任亚锋;河南中医药大学“双一流”创建工程中医学学科项目(HSRP-DFCTCM-2023-1-25),项目负责人:任亚锋
Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury
Guo Jia1, 2, Ren Yafeng2, Li Bing2, Huang Jing1, Shang Wenya1, Yang Yike1, Liu Huiyao1
- 1School of Rehabilitation Medicine, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2024-04-08Accepted:2024-04-24Online:2025-12-28Published:2025-03-14 -
Contact:Ren Yafeng, MD, Chief physician, Master’s supervisor, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Guo Jia, Master candidate, School of Rehabilitation Medicine, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:Henan Province Traditional Chinese Medicine Science Research Special Project, No. 2021JDZY022, 2022JDZY015 (to RYF); “Double First Class” Creation Engineering Traditional Chinese Medicine Discipline Project of Henan University of Chinese Medicine, No. HSRP-DFCTCM-2023-1-25 (to RYF)
摘要:
文题释义:
脊髓损伤:是一种极其严重的创伤性疾病,会导致患者感觉、运动以及自主神经等功能障碍,由于其病理过程高度复杂,目前尚无明确有效的临床治疗策略。间充质干细胞来源的外泌体:是由间充质干细胞分泌的细胞外囊泡之一,是一种新型的细胞间通讯工具,已被用作局部或全身递送miRNA的生物载体,以治疗包括脊髓损伤在内的多种疾病。而脊髓损伤会诱导miRNA的异常表达,引起继发性损伤反应。越来越多的证据表明,miRNA与脊髓损伤的发病机制有关。
摘要
背景:目前,脊髓损伤给患者和国家医疗服务带来巨大的负担,有关于脊髓损伤预防、治疗和康复已成为医学领域的一个重要课题。因此,在深入了解脊髓损伤潜在分子机制的基础上,探索新的有效治疗策略具有重要意义。
目的:综述负载多种miRNA的间充质干细胞源外泌体在改善脊髓损伤功能方面作用机制的研究进展,并基于临床转化现状,对其投入临床使用提出几点思考和展望。
方法:由第一作者检索中国知网和PubMed数据库,分别以“间充质干细胞,外泌体,脊髓损伤,miRNA,病理生理学,临床转化,临床试验,良好生产规范”及“mesenchymal stem cells,exosome,spinal cord injury,miRNA,pathophysiology,clinical translation,clinical trial,good manufacturing practice”为中英文检索词检索相关文献。文献类型包括论著和综述,语言种类为英文和中文,最终筛选出72篇文献进行综述分析。
结果与结论:①文章概述了外泌体的生物特性和其可作为负载miRNA良好载体的优势。由间充质干细胞源外泌体介导的多种miRNA主要通过调节神经再生相关蛋白的表达、抑制RAS同源基因家族成员A、激活环磷腺苷效应元件结合蛋白和信号传导及转录激活蛋白3、调节磷酸酯酶与张力蛋白同源物/程序性细胞死亡因子4通路等多个方面来促进神经元功能的恢复;通过调节内质网到细胞核信号1、干扰素调节因子5的表达、Toll样受体4/核转录因子kB通路、下调相关促炎因子等方面来改善炎症反应;抑制含发芽相关的EVH1域1和磷酸肌醇3激酶调节亚基2来促进血管生成。②进一步对比分析发现,miR-216-5p,miR-145-5p及miR-146b均通过调节相关通路来改善炎症反应,将这些miRNA联合运用可能会产生更显著效果;缺氧预处理可能是一种增加外泌体治疗效果的预处理方法。③目前尚无将间充质干细胞源外泌体应用于脊髓损伤的临床试验,这与其投入临床使用前需满足良好生产规范有关;现阶段需要大规模大批量的细胞生产、高效统一分离外泌体方法的缺失以及在投入临床使用前需要通过严格的监管审批机制等诸多因素阻碍了其临床应用。④miRNA作为间充质干细胞源外泌体内容物在治疗脊髓损伤中具有巨大潜力,未来应深入探索其作用机制以及加快推动进入临床试验阶段,为治疗脊髓损伤提供切实有效的新方法。
中图分类号:
引用本文
郭 佳, 任亚锋, 李 冰, 黄 靖, 尚文雅, 杨溢珂, 刘慧瑶. 负载miRNA间充质干细胞源外泌体改善脊髓损伤的作用机制[J]. 中国组织工程研究, 2025, 29(36): 7827-7838.
Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838.
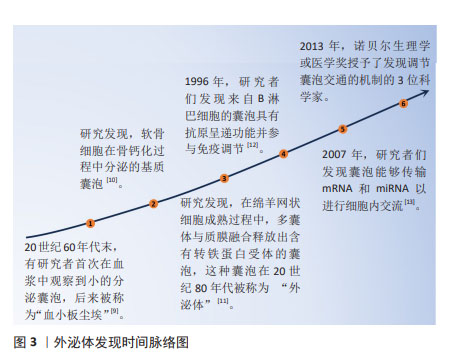
2.1.1 外泌体的发现 长期以来,细胞外囊泡被认为是细胞排泄代谢废物的“垃圾袋”。20世纪60年代末,有研究者首次在血浆中观察到小的分泌囊泡,后来被称为“血小板尘埃”[9]。与此同时,有研究者发现了软骨细胞在骨钙化过程中分泌的基质囊泡[10]。还有研究者发现,在绵羊网状细胞成熟过程中,多囊体与质膜融合释放出含有转铁蛋白受体的囊泡,这种囊泡在20世纪80年代被称为“外泌体”[11]。然而,直到1996年有研究者发现来自B淋巴细胞的囊泡具有抗原呈递功能并参与免疫调节时,外泌体才被认为是一个重要的东西[12]。另一个重大发现发生在2007年,当时人们发现囊泡能够传输mRNA和miRNA以进行细胞内交流[13]。在2013年,诺贝尔生理学或医学奖被授予了3位科学家(James E. Rothman,Randy W. Schekman和Thomas C. Südhof),以表彰他们“发现了调节囊泡交通的机制,囊泡交通是细胞中的一个主要运输系统”。从那时起,外泌体研究便成为一大热点,研究者利用其独特生物特性在治疗疾病的过程中发挥出了巨大效力,具体时间脉络见图3。
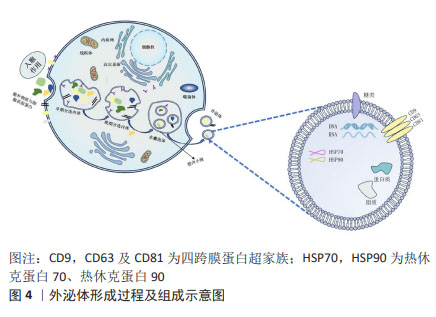
2.1.2 间充质干细胞-外泌体的形成 在间充质干细胞-外泌体的形成过程中,涉及质膜的两次内陷和含有腔内小泡的细胞内多囊泡体的形成。最初,早期分选内体是由细胞膜第一次内陷,即膜的内吞和向内发芽形成的,其中含有细胞外成分和细胞表面蛋白。然后,早期分选内体经高尔基体、内质网和线粒体成熟形成晚期分选内体,并最终通过质膜的二次内陷产生含有腔内小泡的多囊泡体。部分多囊泡体可以与溶酶体或自噬体融合以被降解,或者与质膜融合以释放被包含的腔内小泡来作为外泌体[14]。
研究发现,外泌体可通过受体配体结合、直接膜融合和内吞作用将其内容物运送到靶细胞[15],是细胞间通讯的重要介质,在生理和病理过程中发挥着重要作用。
2.1.3 间充质干细胞-外泌体的内容物及功能 间充质干细胞-外泌体包含蛋白质、脂质以及糖类和核酸等多种物质[16]。即使是来自同一个细胞,外泌体的大小和所携带的物质也各不相同;不过,在不同来源的外泌体中发现了一些部分共同的物质[17]。
外泌体中不仅有取决于分泌细胞类型的特定蛋白质,还有一个无论细胞类型如何,均存在的特定的蛋白质子集。常见蛋白有:对细胞靶向和黏附至关重要的四跨膜蛋白超家族(CD9,CD63及CD81)、具有分子伴侣特性的热休克蛋白(HSP70,HSP90)和其他相关蛋白的蛋白质,这些物质通常用于外泌体的鉴定[18]。
外泌体富含特定的脂质,主要包括神经酰胺、胆固醇和鞘脂,其有助于外泌体的形成和结构稳定性[19]。外泌体还含有表面多糖和聚糖,它们存在于其质膜上,有助于外泌体与受体细胞的对接和附着[20]。此外,外泌体携带DNA和RNA,包括mRNA和一些非编码RNA。这些RNA可调节细胞间通讯,特别是miRNA,在各种生物机制中发挥着至关重要的作用。外泌体的形成过程及组成示意图见图4。
2.1.4 间充质干细胞-外泌体负载miRNA 目前,miRNA在治疗脊髓损伤方面展现出巨大潜力,但活细胞分泌量较少,体内运输的便捷性有限,从而对其治疗效果产生不利影响。因此,如何将miRNA运送到目标细胞或组织仍然是其临床应用面临的巨大挑战。
外泌体被认为是向中枢神经系统递送miRNA的理想载体,其可作为治疗脊髓损伤的理想miRNA载体的优势如下:首先,外泌体可穿过几乎98%的全身用药都无法通过的血脑屏障;其次,外泌体具有脂质双层结构,因此可以有效保护其内容物,免受酶分解或其他过程的影响;最后,作为miRNA载体的外泌体能靶向输入受体细胞。在特异性表达miRNA的细胞中,该细胞的外泌体更有可能含有该特异性miRNA,这一特性对于提取目标外泌体-miRNA和研究携带miRNA的外泌体在疾病中的作用具有积极作用[21]。
2.2 脊髓损伤的病理生理学机制 脊髓损伤的病理生理变化主要包括两个阶段:原发性损伤和继发性损伤。原发性损伤包括脊髓出血、神经细胞膜破裂以及外部牵引或压迫引起的血脑屏障破坏[22]。继发性损伤是原发性损伤后的一系列连锁反应,包括局部血流障碍、组织缺血缺氧、炎性细胞浸润和神经细胞坏死,进而引发自由基形成、谷氨酸介导的兴奋毒性和神经毒性。继发性损伤会导致脊髓损伤区域进一步扩大,更多神经细胞死亡,甚至神经纤维变形[23]。一般来说,继发性损伤比原发性损伤更严重,更难处理。因此,治疗脊髓损伤的重点应放在如何促进轴突再生、减少炎症和重塑功能性神经回路上。
2.3 间充质干细胞-外泌体所负载miRNA在治疗脊髓损伤中的作用机制 大量研究表明,间充质干细胞-外泌体装载的miRNAs可以通过多个方面来促进脊髓损伤后的恢复,现将关于负载多种miRNA的间充质干细胞-外泌体对脊髓损伤的治疗作用大致分为3类加以阐述:①促进神经功能恢复:促进神经元再生与轴突再生和抑制凋亡;②调节炎症反应:调节巨噬细胞、小胶质细胞和星形胶质细胞的表型和下调促炎因子的表达;③促进血管生成。
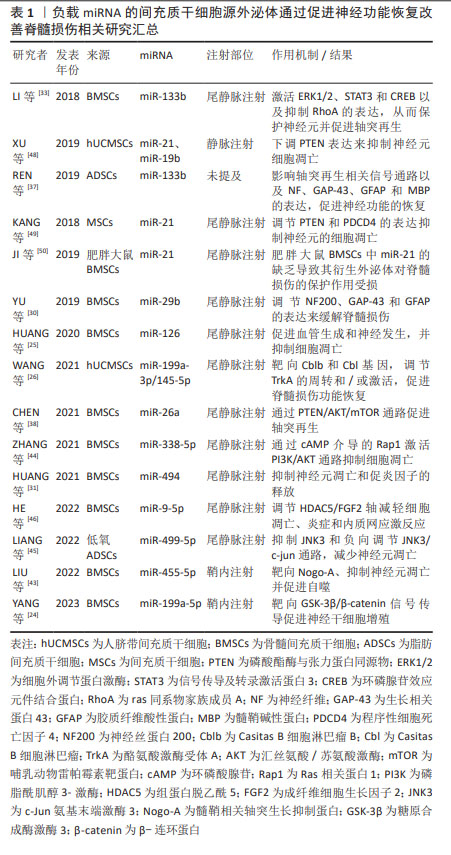
2.3.1 促进神经功能恢复 由间充质干细胞-外泌体所运输的miRNA对神经功能恢复的促进作用已被大量临床前实验证实,并预示着未来可能用于临床治疗中,其中包括 miR-199a-5p,miR-199a-3p/145-5p,miR-29b,miR-133b以及miR-26等多种miRNA。
促进神经元再生:在促进神经元再生方面,YANG等[24]通过建立使用神经干细胞的氧-葡萄糖剥夺/再氧合模型和大鼠主动脉夹闭模型,来探究骨髓间充质干细胞-外泌体对神经干细胞增殖的影响和其潜在机制。研究发现,骨髓间充质干细胞-外泌体可促进神经干细胞的增殖,并且这种有益作用是由骨髓间充质干细胞-外泌体直接转运成熟miR-199a-5p产生的,而后证实糖原合成酶激酶3(GSK-3β)是miR-199a-5p的直接靶标,因此,得出miR-199a-5p可通过靶向调节GSK-3β促进神经干细胞增殖。HUANG等[25]实验指出骨髓间充质干细胞-外泌体来源的miR-126增加了内源性神经干细胞的两个标记物巢蛋白(Nestin)和SRY-box转录因子2(SOX2)的表达,同时也增加了神经元细胞数量,从而促进脊髓损伤后的内源性神经发生。内源性神经发生可从神经干细胞中生成新的神经细胞类型,这对损伤后神经功能的恢复非常重要。
WANG等[26]通过研究源自人脐带间充质干细胞的miR-199a-3p/145-5p在体内和体外诱导神经元生长的潜在作用机制,发现Casitas B细胞淋巴瘤B(Cblb)是miR-199a-3p的直接靶标,Casitas B细胞淋巴瘤(Cbl)是miR-145-5p的直接靶标。在体外可通过调节神经生长因子/酪氨酸激酶受体A(TrkA)通路促进受脂多糖抑制的PC12细胞分化。在体内实验发现,外泌体-miR-199a-3p/145-5p能上调损伤部位的TrkA表达,促进脊髓损伤大鼠的运动功能。因此得出,人脐带间充质干细胞-外泌体-miR-199a-3p/145-5p可通过影响TrkA泛素化和促进神经生长因子 /TrkA信号通路,成为治疗神经元损伤的有效策略。而神经生长因子/TrkA这一信号通路在神经系统的发育和成熟过程中不可或缺。
促进神经元再生这一功能还可通过调节神经再生相关蛋白的表达来实现。神经丝蛋白200(NF200)是神经细胞和轴突的骨架结构[27]。生长相关蛋白43(GAP-43)是脊椎动物神经细胞膜上的特异性磷蛋白,被认为是突触可塑性、神经元发育和再生的标志物[28]。胶质纤维酸性蛋白是构成神经胶质细胞的主要成分的一种细胞骨架蛋白[29],这3种蛋白可以反映脊髓损伤大鼠脊髓组织中神经元再生的程度。YU等[30]在使用标准打击装置来制备脊髓损伤模型的SD大鼠中,通过尾静脉注射从miR-29b改良的大鼠胫骨和股骨骨髓间充质干细胞中获得的外泌体,结果发现,外泌体可减少胶质纤维酸性蛋白和收缩神经细胞数量,增加神经丝蛋白200和生长相关蛋白43的表达、提高神经元再生率和Basso Beattie Bresnahan(BBB)评分以及运动功能,从而促进神经元再生。此外,HUANG等[31]通过化学转染将miR-494有效地装载到外泌体中,通过尾静脉注射外泌体-miR-494发现其下调了胶质纤维酸性蛋白的表达,并促进神经纤维增加,提高大鼠BBB评分,从而促进了神经细胞的再生与行为功能的恢复。
促进轴突再生与抑制凋亡:神经元轴突在传递生物信号方面起着核心作用,但它们在损伤后无法接收和发送电子和化学信号。即使脊髓损伤患者的运动能力得到了一定程度的恢复,但损伤的轴突很少能像正常脊髓中的轴突那样重新连接,患者的运动功能也无法恢复正常[32]。因此,促进轴突再生与恢复对脊髓损伤患者的运动恢复至关重要。已有研究证实,中枢神经系统损伤后轴突不能再生的主要原因是抑制分子的存在,包括少突胶质细胞髓鞘糖蛋白、髓鞘-Nogo和髓鞘相关糖蛋白。
miR-133b在促进轴突生长方面被广泛研究。在SD大鼠动脉瘤夹闭合性脊髓损伤模型中,尾静脉注射外泌体-miR-133b后,可减少ras同系物家族成员A(RhoA)表达、神经元凋亡和病变面积,同时增加神经元再生能力、成熟神经元数量、生长相关蛋白43、神经纤维及神经元核(NeuN)等物质的表达和促进神经元生长以及后肢运动功能的恢复。因此得出,miR-133b可通过靶向抑制RhoA,促进细胞外调节蛋白激酶(extracellular signal-regulated kinase 1/2,ERK1/2)的磷酸化来进行脊髓损伤后的神经保护作用[33]。RhoA是Rho家族的成员之一,可作用于其直接下游效应物Rho相关激酶(Rho kinase,ROCK)。RhoA/ROCK信号通路在急性脊髓损伤后脊髓神经元的死亡中起着关键作用[34]。而ERK1/2的激活可保护神经元免受凋亡,并改善脊髓损伤后的功能恢复[35]。同时,研究也表明,其也可促进环磷腺苷效应元件结合蛋白(cAMP-response element binding protein,CREB)和信号传导及转录激活蛋白3(signal tranducers andactivators of transcription 3,STAT3)的磷酸化,增强脊髓损伤后轴突的再生能力。CREB的激活足以克服髓鞘相关抑制剂,并促进体内脊髓轴突的再生[36]。而REN等[37]也利用miR-133b修饰的脂肪间充质干细胞-外泌体对脊髓损伤后神经功能的恢复作用及其机制进行探究,结果发现miR-133b修饰的脂肪间充质干细胞外泌体可通过下调RhoA、促进CREB和STAT3磷酸化以及改变神经纤维、生长相关蛋白43、胶质纤维酸性蛋白和髓鞘碱性蛋白(MBP)的表达,促进脊髓损伤大鼠轴突的恢复。上述两项实验均利用miR-133b为外泌体内容物,并且得到相似结果,但其来源不一致,为探究何种间充质干细胞为用于递送miR-133b的外泌体治疗最佳来源,可在后续实验中进一步对比探究,为基于间充质干细胞-外泌体的治疗提供新思路。
CHEN等[38]研究发现外泌体-miR-26a能够上调神经纤维和?Ⅲ微管蛋白的表达,从而促进轴突再生。磷酸酯酶与张力蛋白同源物(phosphatase and tensin homolog deleted on chromosome 10,PTEN)被认为是哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路的一个重要负调控因子,它调控轴突再生、神经元存活和中枢神经系统损伤后的功能恢复。缺失或抑制PTEN可激活mTOR信号传导,从而在神经系统损伤后发挥神经保护作用[39]。在该研究中,将外泌体-miR-26a注入脊髓损伤大鼠的尾静脉,进一步探究发现其可下调PTEN的水平并激活mTOR通路。因此,过表达miR-26a的外泌体可通过PTEN/汇丝氨酸/苏氨酸激酶(AKT)/mTOR通路发挥脊髓损伤后的神经保护作用。
而神经细胞凋亡是脊髓损伤过程中另一个主要的继发性损伤机制[40]。Bcl-2相关X蛋白(Bax)和B淋巴细胞瘤-2基因(Bcl-2)是最常见的细胞凋亡标志物,分别具有促凋亡和抗凋亡作用[41]。胱天蛋白酶3(Caspase-3)是脊髓神经凋亡中最重要的半胱氨酸蛋白酶,抑制caspase 3的活性可减轻脊髓继发性损伤[42]。HUANG等[25]用来自间充质干细胞-外泌体的miR-126处理脊髓损伤大鼠模型后,发现其可抑制Bax和裂解的caspase-3的表达,增加Bcl-2的表达,同时显著减少脊髓中TUNEL阳性细胞的数量,因此表明其具有抗脊髓损伤后细胞凋亡的作用。LIU等[43]发现,鞘内注射经miR-455-5p慢病毒载体转染的骨髓间充质干细胞-外泌体能显著减少模型大鼠脊髓神经元的凋亡,阻断caspase3的裂解。同时也下调了其靶基因髓鞘相关轴突生长抑制蛋白(Nogo-A),从而抑制神经元凋亡来促进神经保护。然而,关于骨髓间充质干细胞-外泌体-miR-455-5p/Nogo-A轴在脊髓缺血再灌损伤中的作用还有待进一步深入细致的研究。
ZHANG等[44]在大鼠脊髓挫伤模型中发现,过表达miR-338-5p的外泌体能显著提高神经丝蛋白M(NF-M)和生长相关蛋白43的表达水平,降低髓鞘相关糖蛋白和胶质纤维酸性蛋白的表达水平,从而提供神经保护作用。在体外研究中,过表达外泌体-miR-338-5p抑制了PC12细胞在过氧化氢诱导的氧化应激损伤后的细胞凋亡。进一步研究得出,miR-338-5p可下调其靶标大麻素受体1基因(Cnr1)的表达,来激活Ras相关蛋白1(Rap1),增加环磷酸腺苷(cAMP)的积累,随后激活磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/丝氨酸/AKT通路,从而抑制细胞凋亡,提高神经元存活率。
为模拟脊髓损伤后的缺氧环境,LIANG等[45]在体外建立了神经元氧-葡萄糖剥夺和再灌注模型,观察到低氧条件下的外泌体能显著减少神经元的凋亡;同时,向脊髓损伤大鼠的尾静脉注射缺氧处理的外泌体,发现其能显著改善后肢功能的恢复,并减少受损部位空洞的形成。利用miRNA序列分析及一系列实验确定miR-499-5p在其中发挥了作用,随后利用一系列生物信息学分析工具确定miR-499-5p的靶基因c-Jun氨基末端激酶3(JNK3),从而提出miR-499-5p通过靶向抑制JNK3来负调控JNK3/c-jun凋亡信号通路,来调控神经元凋亡。值得一提的是,此项研究中透射电子显微镜和纳米颗粒跟踪分析技术结果显示,外泌体和低氧外泌体在大小、形状和电子密度方面没有形态学差异。此外,脂肪间充质干细胞分泌的外泌体颗粒数量也无显著差异,这表明缺氧环境主要是通过改变外泌体的组成而不是增加外泌体的分泌来产生不同的效应。并且,这项实验也并不能排除其他基因单独或与低氧外泌体联合发挥治疗作用的可能性,因为在低氧预处理过程中,有多种miRNA含量均上调,有可能是多种miRNA一齐发挥了作用。
HE等[46]探讨了骨髓间充质干细胞-外泌体-miR-9-5p对脂多糖诱导的PC12细胞凋亡、炎症和内质网应激的影响及调控机制,并进一步研究其对脊髓损伤大鼠的影响。在体外实验中发现,外泌体中miR-9-5p的表达增加,并且抑制了细胞凋亡以及炎症细胞因子和内质网应激标志蛋白的水平。在脊髓损伤大鼠模型中,发现miR-9-5p治疗缓解了脊髓损伤大鼠的运动能力、组织病理学损伤、神经元凋亡。并且,进一步研究确定组蛋白脱乙酰5(Histone deacetylase 5,HDAC5)是miR-9-5p的靶基因。而PC12细胞中成纤维细胞生长因子2的表达受HDAC5介导的去乙酰化调控。因此,骨髓间充质干细胞-外泌体-miR-9-5p通过抑制HDAC5介导的去乙酰化来促进成纤维细胞生长因子2的表达,从而抑制脂多糖诱导的PC12细胞凋亡、炎症和内质网应激。
而作为在染色体10q24上新发现的肿瘤抑制基因,程序性细胞死亡因子4(programmed cell death 4,PDCD4)也与细胞的程序性死亡有关[47]。XU等[48]的研究表明从人脐带间充质干细胞-外泌体中提取的miR-21和miR-19b可通过下调PTEN的表达来抑制神经元的凋亡。而KANG等[49]进一步研究得到,PDCD4和PTEN是miR-21的直接靶标。从转染的间充质干细胞上清液中收集的外泌体中含有miR-21,它能改善脊髓损伤大鼠的功能恢复,并通过miR-21/PTEN/PDCD4信号通路抑制神经元细胞死亡。JI等[50]则是利用肥胖大鼠间充质干细胞作为外泌体来源,发现其中miR-21水平下降,并且由于肥胖大鼠间充质干细胞的胰岛素抵抗作用,使其对脊髓损伤的保护作用受损,从侧面进一步证实了miR-21作为脊髓损伤治疗靶点的潜力。以上研究表明,含有miR-21的外泌体很有可能是治疗脊髓损伤的一种潜在方法。
上述内容介绍了间充质干细胞-外泌体所递送的miRNA可通过促进神经元生成、促进轴突再生以及抑制凋亡3个方面来促进脊髓损伤后神经功能的恢复。这其中有两点值得引起注意:第一,同一外泌体-miRNA在脊髓损伤的不同阶段可能具有不同的功能,如miR-126同时具有促进内源性神经生成、减少凋亡以及促进血管生成的作用。当上调外泌体中相关miRNA的含量时,可能对脊髓损伤某一方面功能的改善具有重要的治疗价值。第二,虽然肥胖并不影响从间充质干细胞中分离的外泌体数量与分布,但却改变了它们的miRNA载体。还需要更多的实验来确定肥胖大鼠来源的间充质干细胞中整个miRNA特征的变化,并探讨这些变化的意义。
2.3.2 调节炎症反应 神经炎症是中枢神经系统先天免疫系统响应炎症挑战的激活,其特征在于中枢神经系统内的大量细胞和分子变化。这种炎症由细胞因子/趋化因子的上调介导,细胞因子/趋化因子由常驻小胶质细胞、星形胶质细胞、外周来源的免疫细胞等一系列细胞产生[51]。目前,对于脊髓损伤后炎症机制的研究不断深入发展,合理调控炎症细胞以及促炎、趋化因子的发展可在一定程度上减少脊髓损伤后组织的损伤。
调控小胶质细胞/巨噬细胞与星形胶质细胞表型:作为炎症反应过程中的关键效应细胞,小胶质细胞/巨噬细胞激活在脊髓损伤后神经炎症的激活和调节中发挥着重要作用[52]。其对神经炎症和神经发生具有双重作用,这取决于它们的极化程度:经典的M1表型分泌促炎细胞因子,加剧神经元的损伤;相反,M2表型分泌抗炎细胞因子,以减轻神经元损伤并有利于组织修复[53]。因此,应重点探索将小胶质细胞/巨噬细胞从M1表型转变为M2表型以及抑制有害的过度神经炎症的治疗策略,为脊髓损伤的治疗提供新的治疗途径。
LI等[54]建立了脊髓缺血再灌注损伤大鼠模型,给大鼠注射含miR-124-3p的外泌体,并将巨噬细胞与含miR-124-3p的骨髓间充质干细胞-外泌体共同培养。随后测定抑制内质网到细胞核信号1(Ern1)与巨噬细胞极化相关标记物的表达。研究发现,来自骨髓间充质干细胞-外泌体的miR-124-3p通过抑制Ern1并促进M2巨噬细胞极化,改善了脊髓缺血再灌注损伤及其相关的神经损伤。然而,要阐明Ern1如何调控巨噬细胞极化以及其他miRNA是否能调控Ern1,还需要进一步研究。CHANG等[55]建立大鼠脊髓损伤模型并用提取的骨髓间充质干细胞-外泌体治疗,发现其促进了大鼠运动功能的恢复和M2表型的极化,同时抑制了脊髓损伤引起的神经元凋亡、变性和炎症反应。随后证明干扰素调节因子5(Interferon regulator factor 5,IRF5)的变化与治疗效果的关联性。进一步综合生物信息学预测确定,miR-125a是IRF5的上游miRNA。并且外泌体中miR-125a的敲除增加了IRF5在组织中的表达,削弱了骨髓间充质干细胞-外泌体对脊髓损伤的神经保护作用。因此得出,来源于骨髓间充质干细胞-外泌体所负载miR-125a靶向并负向调节IRF5的表达,促进了M2巨噬细胞的极化,从而改善脊髓损伤。IRF5可激活炎症细胞因子和肿瘤抑制因子等基因。M1型巨噬细胞的特点是富集表达IRF5,而在M2型中强化表达IRF5则会增强M1型特异性细胞因子和趋化因子的表达[56]。因此,miR-125a可通过增强M2极化被视为治疗脊髓损伤的新靶点。然而,还需要进一步的研究来阐明脊髓损伤中的下游信号轴。
对于小胶质细胞的调控,LIU等[57]研究表明,缺氧外泌体能在体内和体外将小胶质细胞极化从M1表型转变为M2表型,从而促进行为功能的恢复。通过miRNA阵列分析,确定miR-216a-5p参与了缺氧外泌体介导的小胶质细胞极化。随后确定Toll样受体4(Toll-like receptor 4,TLR4)为miR-216a-5p的下游靶基因,一系列功能增益和缺失实验证实了miR-216a-5p/TLR4轴。最后,指出来自缺氧骨髓间充质干细胞-外泌体可以通过转移miR-216a-5p抑制TLR4/核转录因子kB和激活PI3K/AKT信号通路,使小胶质细胞从M1促炎表型转变为M2抗炎表型,从而改善脊髓损伤。然而,该实验并没有解释TLR4/核转录因子κB和PI3K/AKT信号通路之间相互影响的内在机制,还需要进行下一步的实验,并且,也不能排除其他可能单独或与缺氧骨髓间充质干细胞-外泌体结合发挥作用以显示治疗效果的基因。
同时,这也揭示低氧预处理是优化间充质干细胞来源的外泌体治疗作用的一种有前途和有效的方法。但是,这项研究只揭示了一种可能性,即在缺氧条件下,骨髓间充质干细胞-外泌体可能会导致miR-216a-5p的增加,从而进一步帮助加强脊髓损伤后的功能恢复。XUE等[58]则进一步探索骨髓间充质干细胞-外泌体衍生的miR-216a-5p在脊髓损伤中的直接参与作用。他们发现骨髓间充质干细胞-外泌体衍生的miR-216a-5p能减轻脊髓损伤中神经元损伤和小胶质细胞介导的炎症反应,从而促进功能恢复,这可能归因于它对TLR4/核转录因子κB通路的抑制作用。仍需要更多的证据来验证这项发现。
ZHANG等[59]收集了骨髓间充质干细胞-外泌体来处理脊髓损伤大鼠和脂多糖诱导的小胶质细胞,发现其在体内体外均可降低促炎因子的表达,升高miR-181c含量,并产生抗炎作用。为深入探索其机制,又进一步确定了其靶基因PTEN,并验证了骨髓间充质干细胞-外泌体可通过其所含的miR-181c抑制PTEN和核转录因子κB信号来改善脊髓损伤。
星形胶质细胞是中枢神经系统中数量最多的细胞,具有神经营养、血流调节和其他平衡维持等功能。急性损伤会引发小胶质细胞活化和免疫细胞浸润等严重的炎症反应,进而推动星形胶质细胞的反应性[60]。反应性星形胶质细胞可通过促进组织碎片清除和抑制过度炎症反应来保护神经。然而,中枢神经系统损伤也会诱导星形胶质细胞获得神经毒性表型,从而对神经组织造成相应的过度损伤[61]。因此,逆转神经毒性表型的获得或消除神经毒性星形胶质细胞的有害影响是促进脊髓损伤患者神经功能恢复的重要途径。
作为源自人脐带间充质干细胞-外泌体能显著降低神经毒性星形胶质细胞标记物表达的miRNA之一的miR-146a-5p,在经过一系列功能增益和功能缺失实验后,可证实miR-146a-5p修饰的外泌体通过抑制典型核转录因子κB通路激活过程中的信号转导介质肿瘤坏死因子受体相关因子6(Traf6)和白细胞介素1受体相关激酶1(Irak1)的表达,来抑制核转录因子κB信号级联,逆转星形胶质细胞的神经毒性表型,促进脊髓损伤神经功能的恢复。该研究也指出,为获得最佳治疗效果,外泌体应在7 d pi内给药,以减弱星形胶质细胞的神经毒性效应[62]。然而,这项结果并不能排除其他miRNA表现出与miR-146a-5p相同治疗效果的可能性。CHEN等[38]实验发现从miR-26a修饰的间充质干细胞中提取的外泌体减少了炎症反应和星形胶质细胞瘢痕的标志物胶质纤维酸性蛋白的表达和核转录因子κB信号转导。因此,得出miR-26a可通过核转录因子κB通路来调节脊髓损伤中星形胶质细胞的增生。
调控炎症因子:众所周知,先天性免疫细胞和淋巴白细胞在损伤后会被激活,从而导致炎症级联反应。上述炎症细胞会释放各种神经毒素、促炎细胞因子、趋化因子、自由基、兴奋毒性氨基酸、一氧化氮等,为神经元的再生创造一个非常不利的微环境[63]。因此及时调控促炎因子对于治疗脊髓损伤有着重要意义。
LI等[64]在硬膜外压迫脊髓损伤模型的SD大鼠尾静脉注射经miR-544修饰的胫股骨骨髓间充质干细胞-外泌体,发现miR-544明显抑制脊髓损伤后脊髓组织中促炎因子白细胞介素1a、肿瘤坏死因子α、白细胞介素17b和白细胞介素36b的产生,同时提高BBB评分和脊髓神经元存活率,减轻脊髓损伤炎症反应。然而,鉴于外泌体的保护作用,还需要进一步的体外实验。
SHAO等[65]研究发现,miR-137过表达的骨髓间充质干细胞-外泌体可降低脊髓损伤大鼠组织中促炎因子如白细胞介素1β、白细胞介素6和肿瘤坏死因子α等的表达,同时减少组织损伤,提高神经元活力与运动功能的恢复。由于通过鞘内注射将药物直接输送到脊髓的侵入性更大,会对未损伤或损伤的脊髓组织造成毒性,而且在非医院环境中很难进行鞘内注射,该研究选择了尾部静脉注射外泌体。然而,该实验还有一定局限性:尽管在分离外泌体时使用了已被证明高效的分离方法,但在分离后并未评估其纯度。
目前,TLR4/核转录因子κB信号通路是研究脊髓损伤发病相关分子机制最常见的信号通路之一。TLR4是调节宿主免疫系统的关键受体,通过调节核转录因子κB级联诱导炎性细胞因子的表达[66]。
JIANG等[67]研究表明,含有miR-145-5p的骨髓间充质干细胞-外泌体可抑制脊髓损伤大鼠体内TLR4/核转录因子κB通路的激活,从而减轻炎症反应,改善脊髓损伤大鼠的功能恢复,减少组织病理学损伤。同时,miR-145-5p在由脂多糖诱导的PC12细胞中,可特异性靶向TLR4,抑制TLR4/核转录因子κB通路的激活和炎症反应。但是,该实验仍有不足,仅用透射电子显微镜对间充质干细胞中的外泌体进行分析,应该更准确地观察外泌体的结构特征,如利用纳米颗粒追踪分析实验观察颗粒的大小和分布,定量分析异物。WANG等[68]也通过建立体内大鼠脊髓损伤模型和脂多糖刺激的体外细胞模型,证明人脐带间充质干细胞衍生的外泌体miR-146b可通过靶向抑制TLR4和灭活核转录因子κB信号来缓解脊髓损伤和减少炎症细胞因子如白细胞介素1β、白细胞介素6和肿瘤坏死因子α的产生。该实验以肾上腺嗜铬细胞瘤细胞株PC12为研究对象,证明了miR-146b可调控 PC12 细胞的死亡和炎症反应。然而,miR-146b也可能产生于大脑中的其他细胞,如神经元、小胶质细胞和星形胶质细胞,这些细胞产生的miR-146b有何作用,以及miR-146b是否会影响原发性神经元、小胶质细胞、少突胶质细胞和星形胶质细胞的行为,目前仍是未知数。后续应开展进一步的研究,探讨miR-146b对脊髓损伤期间神经元系统的详细影响。
间充质干细胞-外泌体所负载miRNA可通过调节巨噬细胞/小胶质细胞、星形胶质细胞表型和调控促炎因子等方面来改善脊髓损伤后的微环境,促进脊髓损伤恢复。其中,有以下几点值得思考:第一,体内生理条件下的氧浓度与体外培养基中的氧浓度是不同的,体外常氧条件并不能模拟体内真实的缺氧微环境。由于缺氧微环境是包括脊髓损伤在内的各种炎症和病变组织的显著特征,所以应在正常缺氧和缺氧细胞预处理后对这些相互作用进行深入探究,以此来深入发掘脊髓损伤的发病机制,为其治疗提供新思路。第二,TLR4/核转录因子κB这一信号通路在调节炎症过程中反复出现,miR-216-5p,miR-145-5p及miR-146b均能通过调节TLR4/核转录因子κB来发挥作用。由此可见,若集中具有相似作用的miRNA一齐运用于实验,是否可产生更明显的效果来改善脊髓损伤。
2.3.3 促进血管生成 血管生成是脊髓损伤后损伤部位血管演变的基本形式,血运重建在其康复中至关重要。通过提供血液供应充足、触发血管生成和确保血脊髓屏障完整性来针对受损血管系统的干预措施,能够潜在地延缓继发性脊髓损伤发展并促进损伤后的轴突导向和功能恢复[69]。
目前关于促进血管生成这一功能,相关临床前实验较少。HUANG等[25]在将miR-126由骨髓间充质干细胞-外泌体递送到脊髓损伤大鼠模型与体外细胞模型的研究中观察到,在脊髓损伤大鼠模型中,miR-126外泌体能促进脊髓损伤后的血管生成。在体外细胞模型中,可抑制血管内皮生长因子途径的负调控因子含发芽相关的EVH1域1(spouty related EVH domain containing protein1,SPRED1)和磷酸肌醇3激酶调节亚基2(PIK3R2)的表达,从而促进体外人脐静脉内皮细胞的血管生成和迁移,从而促进脊髓损伤后的血管生成。其中,SPRED1可抑制生长因子信号的激活,还能调节肌动蛋白细胞骨架的重组,这对稳定血管非常重要[70]。然而,miR-126富集的外泌体与这两个靶基因之间的确切关系,以及这些过程如何影响脊髓损伤后血管内皮生长因子的表达,还有待进一步明确,需后续实验加以进一步验证。
负载miRNA的间充质干细胞源外泌体通过促进神经功能恢复改善脊髓损伤相关研究汇总见表1。负载miRNA的间充质干细胞源外泌体通过调节炎症反应及促进血管生成改善脊髓损伤相关研究汇总见表2。负载miRNA的间充质干细胞源外泌体治疗脊髓损伤作用机制见图5。

2.4 间充质干细胞-外泌体的临床转化
2.4.1 基于间充质干细胞-外泌体治疗的临床试验 尽管上述大量临床前研究证明了负载miRNA的间充质干细胞-外泌体在治疗脊髓损伤展现的巨大潜力,但是迄今为止,仅有31项基于间充质干细胞-外泌体治疗疾病的临床研究(www.clinicaltrials.gov)。根据研究状态,有4项研究已经完成,剩余研究处于正在招募、未开始招募或未知状态。4项已经完成的将间充质干细胞-外泌体用作治疗的临床研究情况汇总见表3。
并且,有报道中枢神经系统相关疾病的临床试验仅有2项,遗憾的是,其中并无治疗脊髓损伤的相关研究,见表4。
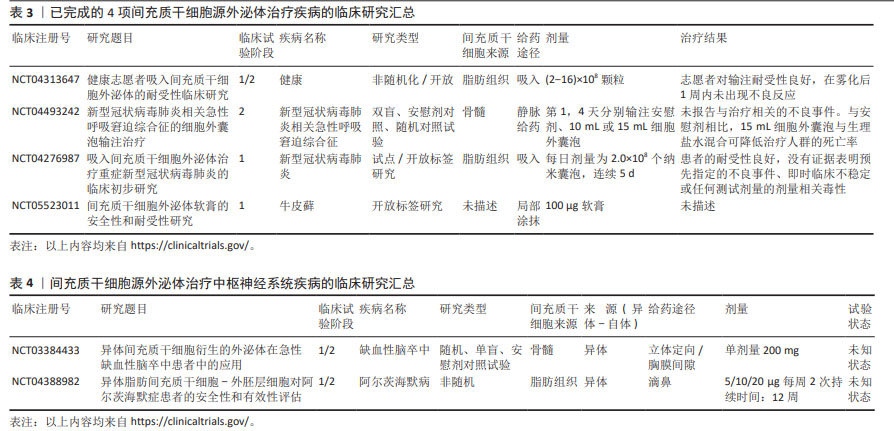
第一项是由ZALI等进行的临床试验(NCT03384433),该试验评估了经miR-124转染的骨髓间充质干细胞-外泌体对急性缺血性脑卒中患者的疗效。在这项1/2期试验中,5例年龄40-80岁的参与者在脑卒中1个月后,在立体定向引导下在缺血区域接受了一次200 mg剂量的转染了miR-124的总蛋白骨髓间充质干细胞-外泌体。在治疗后的12个月内,将对参与者的安全性进行随访,并将其作为主要终点。要测量的参数包括脑卒中复发、脑水肿、癫痫发作和缺血性向出血性转化等副作用的登记情况。此外,潜在疗效还将通过治疗后12个月内操纵兰金量表(Rankin Scale)的改善情况来衡量,并通过0-6分来衡量急性缺血性脑卒中患者的残疾程度。
第二项为由WANG等正在进行的1/2期临床试验(NCT04388982),9例年龄≥50岁的阿尔茨海默病患者接受了异体脂肪间充质干细胞源外泌体治疗,这项试验的主要目的是测试其在治疗由阿尔茨海默病引起的轻度至中度痴呆症方面的疗效。这项研究的参与者分为3组:第1组接受低剂量的间充质干细胞-外泌体,剂量为5 μg,每周滴鼻2次,持续12周;第2组接受中等剂量的间充质干细胞-外泌体,剂量为10 μg,每周滴鼻2次,持续12周;第3组接受20 μg的高剂量间充质干细胞-外泌体,为期12周。主要终点将根据接受治疗的患者中出现肝肾功能异常值以及在3个月内出现不良反应的患者数来衡量。此外,还将在不同时间段内分别通过认知分量表(Alzheimer’s disease assessment scale-cognitive section,ADAS-cog)和阿尔茨海默病协作研究日常生活能力量表(Alzheimer’s disease cooperative study-ADL,ADCS-ADL)评分对患者的认知能力和功能能力进行评估。然而,这两项试验均处于未知状态。
2.4.2 间充质干细胞-外泌体在临床转化中遇到的问题 诚然,间充质干细胞-外泌体从实验室向临床转化的速度缓慢,这主要是由于大规模投入临床中的间充质干细胞-外泌体需要满足良好生产规范(Good manufacturing practice,GMP)。而这意味着在其生产过程中,应从间充质干细胞培养上游、外泌体分离纯化下游、以及表征鉴定等多个方面均需按照良好生产规范的标准来进行,并且在临床应用前,必须对最终产品的质量进行测试来获取监管审批。
间充质干细胞的培养:需要长期及稳定的细胞培养以产生足够数量的外泌体用于临床应用。这就要求在生产过程中应明确定义并验证细胞类型、培养条件、培养系统、解离酶和培养基,以确保一致的产出。并且,应尽量减少人工操作,以防止人为错误并降低污染风险。因此,大规模、自动化和封闭式生产临床级间充质干细胞的系统至关重要,但目前还无法实现,有待后续开发。
外泌体的分离与纯化:由于外泌体与其他污染物之间的大小和密度分布存在串扰,因此,如何分离出高产率、高纯度的外泌体,同时保留其结构和活性仍是一项挑战。常用方法有:速超速离心、切向流过滤法、尺寸排阻色谱法等,但这些方法均存在一定的不足。因此,为获得理想外泌体,可将不同的外泌体分离和纯化方法结合起来,以提高产量和纯度。
建立表征和鉴定方法:包括物理结构和生物活性特征。在将外泌体应用于临床试验之前,确定其生物功能是表征外泌体的主要问题。2018年细胞外囊泡研究基本信息(Minimal information for studies of extracellular vesicles 2018,MISEV2018)准则指出,外泌体应通过透射电子显微镜鉴定为直径为30-150 nm的脂质双层膜囊泡,并表达CD9,CD63和CD81等标记物。定量和单颗粒特征描述应采用包括但不限于颗粒追踪技术和先进流式细胞术的大小和计数方法[71] 。
外泌体生产和临床应用的监管问题亟待解决:遵守监管框架是基于外泌体的疗法获得批准和大规模实施的关键。根据欧洲、美国和澳大利亚的生产和临床试验监管框架,必须提供质量和安全控制数据。因此,在间充质干细胞-外泌体的实验室前期生产过程中就应该考虑到药品的稳定性,将药品送检到第三方机构,来进行药物稳定性与活性测试。
| [1] ALIZADEH A, DYCK SM, KARIMI-ABDOLREZAEE S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. [2] VARMA AK, DAS A, WALLACE G, et al. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013; 38(5):895-905. [3] TYLER JY, XU XM, CHENG JX. Nanomedicine for treating spinal cord injury. Nanoscale. 2013;5(19):8821. [4] YU B, ZHANG X, LI X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142-4157. [5] CHANG C, YAN J, YAO Z, et al. Effects of mesenchymal stem cell-derived paracrine signals and their delivery strategies. Adv Healthc Mater. 2021;10(7):2001689. [6] LIU WZ, MA ZJ, LI JR, et al. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021; 12(1):102. [7] UMEZU T, TADOKORO H, AZUMA K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748-3757. [8] CHEN L, LU FB, CHEN DZ, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93:38-46. [9] NINIVAGGI M, FEIJGE MAH, BAATEN CCFMJ, et al. Additive roles of platelets and fibrinogen inwhole-blood fibrin clot formation upon dilution as assessed by thromboelastometry. Thromb Haemostasis. 2014;111(3):447-457. [10] STRZELECKA-KILISZEK A, BOŻYCKI Ł, KOMIAŻYK M, et al. Vesicles of the intracellular and extracellular transport - key structures in the process of tissue differentiation towards bone and cartilage. Postepy Biochem. 2018;64(3):253-260. [11] JIAO YR, CHEN KX, TANG X, et al. Exosomes derived from mesenchymal stem cells in diabetes and diabetic complications. Cell Death Dis. 2024;15(4):271. [12] POINSOT V, PIZZINAT N, ONG-MEANG V. Engineered and mimicked extracellular nanovesicles for therapeutic delivery. Nanomaterials (Basel). 2024;14(7):639. [13] MCANDREWS KM, XIAO F, CHRONOPOULOS A, et al. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Sci Alliance. 2021;4(9):e202000875. [14] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [15] GUO M, YIN Z, CHEN F, et al. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):109. [16] PEGTEL DM, GOULD SJ. Exosomes. Annu Rev Biochem. 2019;88(1):487-514. [17] MATHIVANAN S, JI H, SIMPSON RJ. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73(10):1907-1920. [18] ZHANG Y, LIU Y, LIU H, et al. Exosomes: biogenesis, biologic function and clinical potential. Cell BioSci. 2019;9(1):19. [19] SUBRA C, LAULAGNIER K, PERRET B, et al. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205-212. [20] BATISTA BS, ENG WS, PILOBELLO KT, et al. Identification of a conserved glycan signature for microvesicles. J Proteome Res. 2011;10(10):4624-4633. [21] BRACCIOLI L, VAN VELTHOVEN C, HEIJNEN CJ. Exosomes: a new weapon to treat the central nervous system. Mol Neurobiol. 2014;49(1):113-119. [22] KUMAR H, ROPPER AE, LEE SH, et al. Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol. 2017;54(5):3578-3590. [23] WHITE-SCHENK D, SHI R, LEARY JF. Nanomedicine strategies for treatment of secondary spinal cord injury. Int J Nanomed. 2015;10:923-938. [24] YANG Y, LI Y, ZHANG S, et al. miR-199a-5p from bone marrow mesenchymal stem cell exosomes promotes the proliferation of neural stem cells by targeting GSK-3β. Acta Biochim Biophys Sin. 2023;55(5):783-794. [25] HUANG JH, XU Y, YIN XM, et al. Exosomes derived from mir-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2020;424:133-145. [26] WANG Y, LAI X, WU D, et al. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther. 2021;12(1):117. [27] GAGLIARDI D, MENERI M, SACCOMANNO D, et al. Diagnostic and prognostic role of blood and cerebrospinal fluid and blood neurofilaments in amyotrophic lateral sclerosis: a review of the literature. Int J Mol Sci. 2019;20(17):4152. [28] CHUNG D, SHUM A, CARAVEO G. GAP-43 and BASP1 in axon regeneration: implications for the treatment of neurodegenerative diseases. Front Cell Dev Biol. 2020;8:567537. [29] KOTLIAROVA A, SIDOROVA YA. Glial cell line-derived neurotrophic factor family ligands, players at the interface of neuroinflammation and neuroprotection: focus onto the glia. Front Cell Neuro Sci. 2021;15:679034. [30] YU T, ZHAO C, HOU S, et al. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res. 2019;52(12):e8735. [31] HUANG W, LIN M, YANG C, et al. Rat bone mesenchymal stem cell-derived exosomes loaded with miR-494 promoting neurofilament regeneration and behavioral function recovery after spinal cord injury. Oxid Med Cell Longevity. 2021;2021:1-15. [32] O’SHEA TM, BURDA JE, SOFRONIEW MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127(9):3259-3270. [33] LI D, ZHANG P, YAO X, et al. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front Neurosci. 2018;12:845. [34] WU X, WALKER CL, LU Q, et al. RhoA/Rho kinase mediates neuronal death through regulating cPLA2 activation. Mol Neurobiol. 2017;54(9):6885-6895. [35] LI F, JIANG Q, SHI KJ, et al. RhoA modulates functional and physical interaction between ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells. Cell Death Dis. 2013;4(7):e708-e708. [36] CHOWDHURY MAR, AN J, JEONG S. The pleiotropic face of CREB family transcription factors. Mol Cells. 2023;46(7):399-413. [37] REN ZW, ZHOU JG, XIONG ZK, et al. Effect of exosomes derived from MiR-133b-modified ADSCs on the recovery of neurological function after SCI. Eur Rev Med Pharmacol Sci. 2019;23(1):52-60. [38] CHEN Y, TIAN Z, HE L, et al. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12(1):224. [39] DU K, ZHENG S, ZHANG Q, et al. Pten deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J NeuroSci. 2015;35(26):9754-9763. [40] ELI I, LERNER DP, GHOGAWALA Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39(2):471-488. [41] WEI AH, ROBERTS AW. BCL2 inhibition: a new paradigm for the treatment of AML and beyond. Hemasphere. 2023;7(6):e912. [42] JULIEN O, WELLS JA. Caspases and their substrates. Cell Death Differ. 2017;24(8): 1380-1389. [43] LIU B, ZHENG W, DAI L, et al. Bone marrow mesenchymal stem cell derived exosomal miR-455-5p protects against spinal cord ischemia reperfusion injury. Tissue Cell. 2022;74:101678. [44] ZHANG A, BAI Z, YI W, et al. Overexpression of miR-338-5p in exosomes derived from mesenchymal stromal cells provides neuroprotective effects by the Cnr1/Rap1/Akt pathway after spinal cord injury in rats. Neurosci Lett. 2021;761:136124. [45] LIANG Y, WU JH, ZHU JH, et al. Exosomes secreted by hypoxia–pre-conditioned adipose-derived mesenchymal stem cells reduce neuronal apoptosis in rats with spinal cord injury. J Neurotraum. 2022;39(9-10):701-714. [46] HE X, ZHANG J, GUO Y, et al. Exosomal miR-9-5p derived from BMSCs alleviates apoptosis, inflammation and endoplasmic reticulum stress in spinal cord injury by regulating the HDAC5/FGF2 axis. Mol Immunol. 2022;145:97-108. [47] MATSUHASHI S, MANIRUJJAMAN M, HAMAJIMA H, et al. Control mechanisms of the tumor suppressor PDCD4: expression and functions. Int J Mol Sci. 2019; 20(9):2304. [48] XU G, AO R, ZHI Z, et al. miR‐21 and miR‐19b delivered by hMSC‐derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol. 2019;234(7):10205-10217. [49] KANG J, LI Z, ZHI Z, et al. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019;26(12):491-503. [50] JI W, JIANG W, LI M, et al. miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie. 2019;167:171-178. [51] HELLENBRAND DJ, QUINN CM, PIPER ZJ, et al. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflamm. 2021;18(1):284. [52] DAVID S, KRONER A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev NeuroSci. 2011;12(7):388-399. [53] ORIHUELA R, MCPHERSON CA, HARRY GJ Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649-665. [54] LI R, ZHAO K, RUAN Q, et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22(1):75. [55] CHANG Q, HAO Y, WANG Y, et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull. 2021;170:199-210. [56] ROBERTS BK, COLLADO G, BARNES BJ. Role of interferon regulatory factor 5 (IRF5) in tumor progression: prognostic and therapeutic potential. Biochim Biophys Acta Rev Cancer. 2024;1879(1):189061. [57] LIU W, WANG J, GE X, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflamm. 2020;17(1):47. [58] XUE H, RAN B, LI J, et al. Bone marrow mesenchymal stem cell exosomes-derived microRNA-216a-5p on locomotor performance, neuronal injury, and microglia inflammation in spinal cord injury. Front Cell Dev Biol. 2023;11:1227440. [59] ZHANG M, WANG L, HUANG S, et al. Exosomes with high level of miR-181c from bone marrow-derived mesenchymal stem cells inhibit inflammation and apoptosis to alleviate spinal cord injury. J Mol Histol. 2021;52(2):301-311. [60] LIDDELOW SA, BARRES BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46(6):957-967. [61] LIDDELOW SA, GUTTENPLAN KA, CLARKE LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481-487. [62] LAI X, WANG Y, WANG X, et al. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res. Ther. 2022;13(1):487. [63] LV B, ZHANG X, YUAN J, et al. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res. Ther. 2021;12(1):36. [64] LI C, LI X, ZHAO B, et al. Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch Physiol Biochem. 2020;126(4):369-375. [65] SHAO Y, WANG Q, LIU L, et al. Alleviation of spinal cord injury by microRNA 137-overexpressing bone marrow mesenchymal stem cell- derived exosomes. Tohoku J Exp. Med. 2023;259(3):237-246. [66] WANG J, ZHANG F, XU H, et al. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin Transl Med. 2022;12(6):e894. [67] JIANG Z, ZHANG J Mesenchymal stem cell-derived exosomes containing miR-145-5p reduce inflammation in spinal cord injury by regulating the TLR4/NF-κB signaling pathway. Cell Cycle. 2021; 20(10):993-1009. [68] WANG X, YANG Y, LI W, et al. Umbilical mesenchymal stem cell-derived exosomes promote spinal cord functional recovery through the miR-146b/TLR4 -mediated NF-κB p65 signaling pathway in rats. Biochem Biophys Rep. 2023;35:101497. [69] TSIVELEKAS KK, EVANGELOPOULOS DS, PALLIS D, et al. Angiogenesis in spinal cord injury: progress and treatment. Cureus. 2022;14(5):e25475. [70] LORENZO C, MCCORMICK F. SPRED proteins and their roles in signal transduction, development, and malignancy. Gene Dev. 2020;34(21-22):1410-1421. [71] CHEN YS, LIN EY, CHIOU TW, et al. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2020;32(2):113-120. [72] XIAO Y, HU X, JIANG P, et al. Thermos-responsive hydrogel system encapsulated engineered exosomes attenuate inflammation and oxidative damage in acute spinal cord injury. Front Bioeng Biotechnol. 2023;11:1216878. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 迟文鑫, 张存鑫, 高 凯, 吕超亮, 张科峰. 川陈皮素抑制BV2小胶质细胞炎症反应的机制[J]. 中国组织工程研究, 2025, 29(7): 1321-1327. |
| [3] | 杨治航, 孙祖延, 黄文良, 万 喻, 陈仕达, 邓 江. 神经生长因子促进兔骨髓间充质干细胞软骨分化并抑制肥大分化[J]. 中国组织工程研究, 2025, 29(7): 1336-1342. |
| [4] | 胡涛涛, 刘 兵, 陈 诚, 殷宗银, 阚道洪, 倪 杰, 叶凌霄, 郑祥兵, 严 敏, 邹 勇. 过表达神经调节蛋白1的人羊膜间充质干细胞促进小鼠皮肤创面愈合[J]. 中国组织工程研究, 2025, 29(7): 1343-1349. |
| [5] | 金 凯, 唐 婷, 李美乐, 谢裕安. 人脐带间充质干细胞条件培养基及外泌体对肝癌细胞增殖、迁移、侵袭和凋亡的影响[J]. 中国组织工程研究, 2025, 29(7): 1350-1355. |
| [6] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [7] | 刘 琪, 李林臻, 李玉生, 焦泓焯, 杨 程, 张君涛. 淫羊藿苷含药血清促进3种细胞共培养体系中软骨细胞增殖和干细胞成软骨分化[J]. 中国组织工程研究, 2025, 29(7): 1371-1379. |
| [8] | 艾克帕尔·艾尔肯, 陈晓涛, 吾凡别克·巴合提. 成骨诱导人牙周膜干细胞来源外泌体促进炎症微环境下人牙周膜干细胞成骨分化[J]. 中国组织工程研究, 2025, 29(7): 1388-1394. |
| [9] | 章镇宇, 梁秋健, 杨 军, 韦相宇, 蒋 捷, 黄林科, 谭 桢. 新橙皮苷治疗骨质疏松症的靶点及对骨髓间充质干细胞成骨分化的作用[J]. 中国组织工程研究, 2025, 29(7): 1437-1447. |
| [10] | 吕丽婷, 于 霞, 张金梅, 高巧婧, 刘仁凡, 李 梦, 王 璐. 脑衰老与外泌体研究进程及现状的文献计量学分析[J]. 中国组织工程研究, 2025, 29(7): 1457-1465. |
| [11] | 翁宗琴, 赵海龙. 外泌体miRNA参与肿瘤化疗耐药的机制[J]. 中国组织工程研究, 2025, 29(7): 1504-1511. |
| [12] | 李佳林, 张耀东, 娄艳茹, 于 洋, 杨 蕊. 间充质干细胞分泌组发挥作用的分子机制[J]. 中国组织工程研究, 2025, 29(7): 1512-1522. |
| [13] | 赵瑞华, 陈思娴, 郭 杨, 石 磊, 吴承杰, 吴 毛, 杨光露, 张昊恒, 马 勇. 温肾通督方促进小鼠脊髓损伤的修复[J]. 中国组织工程研究, 2025, 29(6): 1118-1126. |
| [14] | 何 波, 陈 文, 马岁录, 何志军, 宋 渊, 李金鹏, 刘 涛, 魏晓涛, 王威威, 谢 婧. 皮瓣缺血再灌注损伤的发病机制及治疗进展[J]. 中国组织工程研究, 2025, 29(6): 1230-1238. |
| [15] | 王荣荣, 黄玉珊, 李湘淼, 白金柱. 创伤性脊髓损伤急性期前列腺素E1对血管相关因子的调节和微循环功能的保护[J]. 中国组织工程研究, 2025, 29(5): 958-967. |
间充质干细胞是近年来基于细胞治疗脊髓损伤的一种再生疗法,也是临床试验中最常用的干细胞类型,可以很容易地从成人组织中分离出来,具有多种生物学功能,包括多线分化、促进组织修复、抗炎作用、免疫抑制和神经保护[4]。与胚胎干细胞或诱导多能干细胞不同,从技术上讲,间充质干细胞更适合中国目前的监管框架,伦理争议较少。然而,间充质干细胞在宿主组织中存在存活时间短、分化能力差、致瘤性和免疫排斥等诸多影响其治疗效果的问题[5]。大量研究证实,间充质干细胞的治疗作用主要归因于其通过旁分泌机制分泌的重要分子,如外泌体、趋化因子及生长因子等,而非间充质干细胞自身的移植和分化[6]。
外泌体是细胞外囊泡(EVs)的一种,由多种类型的细胞分泌,通过在细胞间传递miRNA,mRNA,DNA和蛋白质等物质来介导细胞间通信,而无需细胞间的直接接触[7],是优良的生物载体。目前对外泌体的机制研究主要集中在其内容物miRNA和长链非编码RNA的转运方面。miRNA是一类内源性和非编码RNA分子,可以负向调节其目标mRNA的表达,正在成为中枢神经系统损伤的新治疗靶点[6]。通过预转染特异性miRNA质粒,间充质干细胞可以分泌含有高水平特异性miRNA的外泌体[8]。
该文章旨在综述目前有关负载miRNA的间充质干细胞-外泌体促进脊髓损伤功能恢复作用机制的最新研究进展,并且指出目前在临床转化中存在的问题,为其在脊髓损伤中的发展趋势及应用前景发掘新领域。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在2024年1月进行文献检索。
1.1.2 检索文献时限 1990年1月年至2024年1月。
1.1.3 检索数据库 中国知网和PubMed数据库。
1.1.4 检索词 中文检索词为“间充质干细胞,外泌体,脊髓损伤,miRNA,病理生理学,临床转化,临床试验,良好生产规范”,英文检索词为“mesenchymal stem cells,exosome,miRNA,spinal cord injury,pathophysiology,clinical translation,clinical trial,good manufacturing practice”。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以PubMed数据库为例,检索策略见图1。
1.1.8 检索文献数量 初步检索到文献201篇,其中中国知网数据库19篇,PubMed数据库182篇。
1.2 入组标准
1.2.1 纳入标准 ①与负载miRNA的间充质干细胞-外泌体治疗脊髓损伤有关的文章;②与脊髓损伤病理生理机制有关的文章;③与间充质干细胞-外泌体生理特性相关的文章;④与间充质干细胞-外泌体临床转化、临床试验、良好生产规范相关的文章;⑤对相似文章尽量选取发表时间较近者。
1.2.2 排除标准 ①排除内容为间充质干细胞-外泌体的相关研究,但并未涉及miRNA的文章。②排除内容重复的文章;③排除相同研究类型的文章;④排除年限久远的文章。
1.3 文献质量评价与数据提取 文献内容及观点创新,能够体现该领域最新研究进展且科学严谨的文献为重点纳入的文献,与研究内容和目的无关及质量低的不纳入此次研究。第一作者检索得到文献201篇,通过阅读文章题目、摘要进行初步筛选及精读后共纳入72篇文献进行分析,均来自PubMed数据库。文献筛选流程图见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
然而,目前大部分研究都致力于miRNA这一外泌体成分内容物,对其他成分的研究较为有限,因此需要进一步研究以确认外泌体作用。并且,对于多种具有相类似效果的 miRNA,也并没有进一步探索其共同运用将可能产生的效果。对于缺氧以及肥胖等经预处理的间充质干细胞 - 外泌体,预处理并没有改变外泌体物理特征和数量,但是却对治疗效果产生影响,这也值得进一步探究其深层机制。
此外,大多数研究都以啮齿类动物为研究对象。然而,啮齿类动物损伤面积通常较小,人类损伤面积较大,且更为复杂,与啮齿类动物有显著差别。因此,需要进一步扩大研究范围,使用大型动物进行更深入研究。
3.2 作者综述区别于他人他篇的特点 文章主要介绍由间充质干细胞-外泌体介导的多种miRNA在恢复神经功能、调节炎症反应以及促进血管生成等方面改善脊髓损伤作用机制的研究进展,将各项研究结果深入对比,提出几点建议与思考。并且针对目前临床转化现状,提出了当下需要解决的几大问题,有待未来研究者进一步深入探索。
3.3 综述的局限性 该篇综述以间充质干细胞-外泌体中的内容物miRNA为切入点,将其改善脊髓损伤的相关临床前研究的作用机制做了归纳总结,但其中并无临床研究。这是由于间充质干细胞-外泌体若是作为药品投入临床使用中,必须满足良好生产规范下的诸多准则,对于生产过程中的每一步均要求严格,使其进入临床研究的步伐缓慢。因此,目前基于间充质干细胞-外泌体的脊髓损伤临床研究,仍是处于大片空白阶段,需要后续大量实验完善,并与临床前研究结果进行综合分析。
3.4 综述的重要意义 负载miRNA的间充质干细胞-外泌体可通过多个方面来改善脊髓损伤,已被认为是一个很有前景的研究和应用方向。然而在用于临床之前,为提高其安全性与有效性,有关于间充质干细胞-外泌体大规模生产、分离纯化、确定表型特征以及监管审批等问题也成为需要进行严格评估的热点。文章介绍了目前有关负载miRNA的间充质干细胞-外泌体改善脊髓损伤研究的作用机制,并且对其结果进行深入分析,并提出在大量投入临床使用前所需解决的问题,有利于后续研究者深入探索该领域,加快间充质干细胞-外泌体在临床中的应用,从而推动有关治疗脊髓损伤的临床试验研究,为其在脊髓损伤治疗的临床转化中提供全面的理论依据。
3.5 课题组专家的意见和建议 尽管间充质干细胞-外泌体具有治疗脊髓损伤的巨大潜力,但在将间充质干细胞-外泌体疗法用于脊髓损伤的临床试验之前,仍有许多难题需要解决。①应确定间充质干细胞的最佳来源。不同组织来源和不同供体的间充质干细胞具有不同的功能,因此从不同间充质干细胞中分离出的外泌体在其载体和相关特性方面也应存在差异。在上述治疗脊髓损伤的临床前实验中,各团队使用间充质干细胞的种类并不相同,现阶段还缺乏对比不同间充质干细胞-外泌体在治疗中疗效的研究,不同间充质干细胞-外泌体的疗效差异尚未明确。对于脊髓损伤治疗所选用的最佳间充质干细胞- 外泌体可能需要后续大量的临床试验加以验证。②外泌体的储存、保存和运输也是急需解决的问题。冻干技术是一种用于外泌体长期储存的方法,可以有效降低储存和运输成本,并有助于开发高稳定性的产品。然而,冻干处理是否会改变外泌体特性,仍需进一步的研究来证实。③为了确保外泌体在治疗脊髓损伤中的长期效果,必须深入研究注射频率和剂量与其治疗效果之间的关系。同时,还需评估给药方式可能带来的副作用,这对于正确应用外泌体治疗方法至关重要。④大量的证据表明,注射外泌体的治疗能力也受限于其在体内的较短半衰期和较快清除率。因此,如何延缓其在体内的清除,从而延长体内半衰期并提高治疗效力成为目前研究的一大热点。作为一种高含水量的聚合物材料,水凝胶是由交联亲水性聚合物链组成的三维网络,因可吸附水溶液中的大量水分而不发生溶解的特性,已被提出可作为一种策略来联合负载miRNA的间充质干细胞-外泌体[72]。因此,水凝胶可能是与外泌体结合的理想物质,可以延长半衰期、降低清除率,从而提高治疗效果,展现出巨大的潜在治疗效果。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:#br#
脊髓损伤:是一种极其严重的创伤性疾病,会导致患者感觉、运动以及自主神经等功能障碍,由于其病理过程高度复杂,目前尚无明确有效的临床治疗策略。
间充质干细胞来源的外泌体:是由间充质干细胞分泌的细胞外囊泡之一,是一种新型的细胞间通讯工具,已被用作局部或全身递送miRNA的生物载体,以治疗包括脊髓损伤在内的多种疾病。而脊髓损伤会诱导miRNA的异常表达,引起继发性损伤反应。越来越多的证据表明,miRNA与脊髓损伤的发病机制有关。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
目前,脊髓损伤给患者和国家医疗服务带来巨大的心理和经济负担,有关于脊髓损伤预防、治疗和康复已成为医学领域的一个重要课题。因此,在深入了解脊髓损伤潜在分子机制的基础上,探索新的有效治疗策略具有重要意义。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||