中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (28): 6083-6093.doi: 10.12307/2025.467
• 生物材料综述 biomaterial review • 上一篇 下一篇
诱导膜技术在临界尺寸骨缺损修复中的应用:优势与未来发展
李树源1,杨达文1,曾展鹏1,蔡群斌1,张景涛2,周琦石1
- 1广州中医药大学第一附属医院,广东省广州市 510405;2广州中医药大学第一临床医学院,广东省广州市 510405
-
收稿日期:2024-06-21接受日期:2024-07-30出版日期:2025-10-08发布日期:2024-12-07 -
通讯作者:周琦石,博士,主任医师,广州中医药大学第一附属医院,广东省广州市 510405 -
作者简介:李树源,男,1991年生,山西省吕梁市人,汉族,2023年广州中医药大学毕业,博士,主要从事大段骨缺损的修复、中西医结合治疗股骨头坏死研究。 -
基金资助:国家自然科学基金项目(82274540),项目负责人:周琦石
Application of induced membrane technique for repairing critical-sized bone defects: advantages and future development
Li Shuyuan1, Yang Dawen1, Zeng Zhanpeng1, Cai Qunbin1, Zhang Jingtao2, Zhou Qishi1
- 1First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2First School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China
-
Received:2024-06-21Accepted:2024-07-30Online:2025-10-08Published:2024-12-07 -
Contact:Zhou Qishi, MD, Chief physician, First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
About author:Li Shuyuan, MD, First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
Supported by:National Natural Science Foundation of China, No. 82274540 (to ZQS)
摘要:

文题释义:
临界尺寸骨缺损:是指缺损长度超过一定尺寸后,无法自行愈合的骨缺损。目前,“临界”的精确尺寸或体积尚缺乏共识。无论是人还是动物模型,影响骨缺损修复的因素有很多,如:是否存在骨周向性丢失、解剖位置(骨干/干骺端/关节)、软组织条件(包括骨膜损伤)、感染、年龄、损伤机制,以及是否存在慢性疾病和其他合并症等。总之,关于临界尺寸骨缺损的定义仍然需要进一步分类、细化和证实。诱导膜技术:也称Masquelet技术,是一种重建临界尺寸骨缺损的新技术,由法国Masquelet在1986年首先发现并开始应用于临床。该技术主要包括2个步骤:第一期,彻底清除炎症所涉及的骨段,并在残留的骨缺损区域植入聚甲基丙烯酸甲酯(PMMA)骨水泥占位器,来诱导生物膜的形成;第二期,一期术后6-8周,PMMA骨水泥的周围可见诱导膜形成,此时沿原术口切开诱导膜,去除PMMA骨水泥占位器,并将自体或异体骨植入诱导膜中,等待骨缺损愈合。
背景:诱导膜技术(Masquelet技术)是一种两阶段手术重建大段骨缺损的新技术,在临床中应用越来越广泛。然而,该技术修复骨缺损的机制尚不十分明确。
目的:就诱导膜技术的产生背景、修复机制和优势、诱导膜的特征、膜-植骨通讯、动物模型的选择、骨水泥的种类、形貌、所载抗生素对诱导膜的影响、固定方式的选择和骨组织工程材料方面进行综述,以期为未来临界尺寸骨缺损的治疗和诱导膜技术的改进提供新思路。方法:检索 PubMed、Web of Science数据库和中国知网(CNKI)中1986-2024年出版的文献,共检索到890篇参考文献。按与诱导膜技术基础研究相关的入选标准进行人工筛选和分析,排除与主题相关性差和重复的文献,纳入研究的文献包括实验类研究原著、综述、荟萃分析等,最终纳入72篇文献进行归纳和分析。
结果与结论:①诱导膜技术修复骨缺损的机制尚不明确,但膜和植骨两者缺一不可;②诱导膜是一种富含多种骨形成相关细胞、生长因子和血管的分层较明显组织,其血管化和生长因子的分泌随时间而动态变化;③对于动物模型选择,从解剖结构、负重模式和骨重塑的相似度来讲,羊更为接近;从饲养成本和难度、造模周期来讲,大鼠更合适;④聚甲基丙烯酸甲酯(PMMA)骨水泥不是唯一可以用做诱导生物膜的材料,可能有更加合适的材料可以诱导出更高质量的生物膜;骨水泥负载抗生素的剂量(主要是万古霉素)为每40 g的聚甲基丙烯酸甲酯负载1-4 g抗生素;⑤对于动物(特别是大鼠)固定方式而言,钢板使用更为广泛,固定方式更加可靠,可重复性更高;⑥未来可能会有新的材料替代自体骨促进Masquelet技术的骨修复能力。
https://orcid.org/0000-0003-1405-0765 (李树源)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
李树源, 杨达文, 曾展鹏, 蔡群斌, 张景涛, 周琦石. 诱导膜技术在临界尺寸骨缺损修复中的应用:优势与未来发展[J]. 中国组织工程研究, 2025, 29(28): 6083-6093.
Li Shuyuan, Yang Dawen, Zeng Zhanpeng, Cai Qunbin, Zhang Jingtao, Zhou Qishi. Application of induced membrane technique for repairing critical-sized bone defects: advantages and future development[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6083-6093.
1990年,MASQUELET等的第一批病例的惊喜结果鼓励他们进行了基础研究,研究的第一步是确认膜的作用。实验在30只绵羊身上创建了3 cm的股骨节段性缺损,第一步证实了PMMA所诱导的膜可以维持初始植骨不被吸收,并促进骨愈合[4]。第二步组织学和免疫化学研究证实以下结果[5]:①膜有丰富的血管;②内层(靠近水泥)是滑膜状上皮,外层由成纤维细胞、肌成纤维细胞和胶原蛋白组成;③膜分泌生长因子:在第2周观察到高浓度的血管内皮生长因子(VEGF)和转化生长因子β1(TGF-β1),骨形态发生蛋白2(BMP-2)在第4周达到最高水平。这些实验结果支持了临床结果,并鼓励了该技术的更广泛应用和研究。在经历了最初的停滞阶段后,诱导膜技术的研究数量正在持续增加(图3)。当前,诱导膜技术被广泛应用于各个部位的创伤性骨缺损、感染性骨缺损、骨肿瘤切除后骨缺损、先天性胫骨假关节等多种骨缺损的治疗。
2.2 诱导膜技术的修复机制和优势 与骨折修复和牵张成骨不同,植骨到骨愈合的机制并不明确,唯一可以确定的是,膜和植骨都是必要的,两者缺一不可[6-8]。关于诱导膜技术的成功,有3种流行的的理论[9]:首先,膜可以作为物理屏障,阻止负向影响植骨重塑的因子侵入,同时也可以将促再生介质集中在膜内;第2种假设是,膜内的细胞或直接促进骨形成,和/或提供支持植骨重塑的营养因子;第3种假设是,膜内丰富的血管网络已经准备好侵入缺损区,从而在骨坏死和吸收发生之前满足植骨对氧气、细胞、营养物质和废物清除的需要。膜内的细胞、促成骨因子和血管的作用尚未得到直接检验,还需要进行更多的基础和临床的研究。
诱导膜技术的优势有很多[9],该手术依赖于相对标准的外科技术和植入物,因此,在资源匮乏的环境中(例如发展中国家、战地或灾区的野战医院),即使不能完成全程的手术,至少第一阶段手术几乎可以由任何受过骨科培训的外科医生进行。两次手术之间的间隔有利于需要时间解决并发症的情况(如软组织损伤、感染)或需要接受额外化疗(骨肿瘤)的患者。此外,患者高依从性和频繁随访的需要被降低,尤其是使用内固定的情况下。最后,并发症的发生率比牵张成骨低得多。这些优势可能为那些不适合传统植骨或牵张成骨的患者提供保肢选择。
2.3 诱导膜的特征
2.3.1 诱导膜的形态结构 诱导膜的层状结构和分布与骨膜非常相似,报告的膜厚度范围45-2 126 μm不等[6,8,10-14]。
已证实的大鼠3-6周的诱导膜包含3层[8]:①内层与PMMA接触,是一个薄的富含细胞的层,PMMA植入3-6周的所有诱导膜中都观察到该层。②诱导膜的中间层在PMMA植入后3周后逐渐出现,该区域由纤维网络组成,平行于PMMA排列。与内层相比,这部分诱导膜的细胞密度较低,随着时间的推移而增大,提示膜在3-6周之间进行性成熟。③外层接触肌肉,比其他两层厚,位于膜和周围肌肉之间,由疏松杂乱的组织组成,其中包含丰富的血管网络。胶原蛋白网络在3周时较杂乱,但在4-6周的膜上逐渐有组织性(图4)。

2.3.2 诱导膜的组成 对膜的生物学分析往往集中在基质蛋白、生长因子和已知对骨合成代谢有作用的细胞类型上。
(1)细胞因子:由于骨诱导特性,转化生长因子β1是骨愈合过程中的重要生长因子[15-16]。Masquelet 技术的动物模型显示,从第2-6周,诱导膜中发现了高水平的转化生长因子β1[10]。转化生长因子β1的存在表明该生长因子调节诱导膜内的软骨细胞和成骨细胞增殖和分化[17-18]。
骨形态发生蛋白2是属于转化生长因子β蛋白超家族的多功能生长因子。许多骨形态发生蛋白亚型在骨骼和软骨的发育中发挥着重要作用[19]。研究表明,诱导膜中的骨形态发生蛋白2水平在PMMA插入骨缺损区4周时最高,6周时显著下降[10]。这项研究还发现,与骨缺损区的诱导膜相比,骨形态发生蛋白2在皮下植入PMMA所形成膜中的表达水平明显更低,证实了骨环境在诱导膜发育和成骨特性中的作用[10,20-21]。
CBFA1也称Runt相关转录因子2(Runx2),是调节成骨细胞分化的重要转录因子。在动物诱导膜中,接近PMMA间隔物表达CBFA1的细胞数量在手术后2周达到峰值[10,22]。
骨形态发生蛋白2和转化生长因子β1介导SMAD和丝裂原活化蛋白激酶(MAPK)通路后,其表达增加[23-24]。
炎症和抗炎因子,如白细胞介素6、肿瘤坏死因子α、白细胞介素8和白细胞介素10也存在于膜中[25-26],这些因子主要在膜最内层中富集[6-7],并随着膜成熟而下降[25-26](表1)。

(2)诱导膜的血管化:诱导膜是人类和动物模型中不断发育的血管化组织。血管通常在第三层杂乱无章的层(如果存在)或与膜第二层相邻的肌肉中,而不是在内层[8,12-13],血管垂直于PMMA分布[7,10]。在诱导膜形成开始后2周,首先观察到血管化,在4周时血管密度显著增加,在6周时没有进一步变化[10]。LIU等[13]观察到2-8周内诱导膜的厚度和血管密度逐渐增加,8周后,膜的厚度和血管密度逐渐下降。WANG等[18]发现,2周的诱导膜最厚,血管最丰富,4-8周时,诱导膜的厚度和血管数量逐渐下降。
血管内皮生长因子(VEGF)是参与血管生成和调节的生长因子之一。在人和动物的诱导膜中均检测到血管内皮生长因子表达,其表达水平随时间变化,在1个月时浓度较高,在3个月时浓度明显降低[10,25]。AHO等[25]推测衰老诱导膜中的血管分布较少与血管内皮生长因子表达减少之间存在相关性。血管内皮生长因子可以促进内皮细胞的发育,从而在缺氧环境中诱导血管侵袭[27]。因此,血管内皮生长因子的合成和分泌可能是诱导膜发育和功能的一个关键调节过程。血管内皮生长因子在诱导膜形成过程中的角色以及在Masquelet技术骨再生中所起的确切作用仍有必要在后续研究中阐明。血管紧张素Ⅱ(ANG-II)和成纤维细胞生长因子2(FGF-2)作为也参与血管生成的分子,在诱导膜手术后2-8周内被检测到,4周达到峰值,然后开始下降[18]。TANG等[28]证明,具有抗血管生成作用的Notch信号成分[Delta样配体4 (Dll4)和Notch同源物1 (Notch1)]在6周的诱导膜内明显升高,而抑制Dll4/Notch1通路可以促进诱导膜的血管生成。也有研究发现:人诱导膜内的血管内皮生长因子表达并不会随着时间的推移而减少。相反,组织学上可以观察到膜内血管数量的增加[29](表1)。
(3)诱导膜的细胞类型(表1)
免疫细胞:由于膜是免疫反应的产物,因此膜内的免疫细胞(如淋巴细胞和单核细胞/巨噬细胞)已被鉴定出来[14,30-32]。免疫细胞占细胞群的相当大一部分,据报道占组织区域的10%-30%[30,32],这与文献新报道的成骨细胞相关巨噬细胞(有时称为骨巨噬细胞)在体内骨合成代谢和骨折修复中发挥支持作用的观点相吻合[33],并且可以支持诱导膜技术中膜具有营养作用的假说。髓源性抑制细胞最初是在癌症患者中发现的一种免疫细胞,是促进肿瘤进展和转移的主要因素。WANG等[34]在小鼠的诱导膜中也鉴定出了髓源性抑制细胞,其数量增加与膜厚度和毛细血管密度的增加显著相关;髓源性抑制细胞通过T细胞免疫抑制来消除炎症,并通过信号转导与转录激活因子3 (STAT3)信号激活后,产生血管内皮生长因子A、血管紧张素2和缺氧诱导因子1α来促进内皮细胞分化,进而增强诱导膜的血管生成,这对骨修复过程具有有益作用,可能是一种精准治疗的关键靶点。虽然该研究发现膜中的大量髓源性抑制细胞可为后续的骨修复提供血液供应和适当的免疫微环境,但未探讨髓源性抑制细胞募集的机制,也没有继续探讨髓源性抑制细胞募集对后续植骨修复的作用。这一最新发现强调了在研究诱导膜技术时考虑骨折生物学领域之外的细胞类型及其作用的重要性。
血管内皮细胞:前面内容已表述了诱导膜内含丰富的血管网络,血管的形成离不开血管内皮细胞。与纤维状胶原蛋白含量相似,血管密度在最初几周内增加,然后随着膜的老化而下降。
间充质干细胞:是多能基质细胞,可分化成多种细胞类型,包括成骨细胞、软骨细胞、肌细胞和脂肪细胞,并有助于骨愈合过程。GRUBER等[35]对大鼠诱导膜进行免疫组织化学染色首次证实了间充质干细胞(CD34-、CD44+、CD184+细胞)的存在,膜分离的细胞具有成骨和成软骨的能力,该研究还通过对16周的诱导膜进行微阵列分析,发现了许多与干细胞维持相关的基因,如FGF受体1、低密度脂蛋白受体相关蛋白6、BMP受体1A等。WANG等[18]不仅在兔诱导膜中鉴定出CD44 阳性的干细胞,并且发现与2,6和8周的诱导膜相比,4周的诱导膜所分离的间充质干细胞增殖能力更强、碱性磷酸酶活性更高。HENRICH等[10]在PMMA植入2周的大鼠股骨诱导膜的外层以及从膜内提取的细胞中发现了STRO-1+间充质干细胞的存在(STRO-1是骨髓间充质干细胞的一种特异性表面标志物),骨缺损处的诱导膜和骨膜中发现STRO-1+细胞(即间充质干细胞),表明它们可能来源于缺损区附近的骨膜,因为皮下部位的诱导膜并无STRO-1+细胞的存在;作者认为,将间充质干细胞从骨膜吸引到诱导膜的可能机制是基质细胞衍生因子(SDF-1)的趋化作用,虽然并未在3个时间点(2,4和6周)的诱导膜中发现基质细胞衍生因子1的表达,然而这一观察结果并不能排除基质细胞衍生因子1 的存在,因为它有可能在2周测量之前就已经存在。CUTHBERT等[14]首次在人的诱导膜中发现了可进行三系分化的CD271+的骨髓间充质干细胞存在,CD271也是一种干细胞表面标志物,与HENRICH等[10]的研究结果不同,该研究还发现了诱导膜内高表达基质细胞衍生因子1转录物(CXCL12)。基质细胞衍生因子1是一种由间充质干细胞释放的趋化因子,可作用于多种类型的细胞,包括间充质干细胞本身、内皮祖细胞、造血谱系细胞和造血干细胞,该蛋白特异性存在于膜内血管周围区域[36-38]。基于这些发现,研究认为基质细胞衍生因子1参与了间充质干细胞募集到新形成的组织中的自分泌和旁分泌调节途径[14]。GRUBER等[26]也发现6-18周的人诱导膜分离细胞具有成骨、成软骨和成脂分化的能力,微阵列分析发现,与PMMA接触时间的增加导致诱导膜中成骨、干细胞和血管相关基因的显著上调。GESSMANN等[29]在8-113 d的人诱导膜中分离出CD90和CD105阳性的干细胞,且具有成骨、成软骨和成脂分化的能力。综上,干细胞或祖细胞存在于膜内,并可能在第二阶段诱导膜手术开始后,动员、增殖至缺损区并直接促进植骨的修复,间充质干细胞募集与基质细胞衍生因子1的趋化作用有关。
成骨细胞:关于成熟的成骨细胞(定义为碱性磷酸酶或骨钙素阳性细胞)是否存在于膜中仍然不明确。HENRICH等[10]在4周和6周龄的大鼠诱导膜外层的血管周围发现了一些碱性磷酸酶阳性成骨细胞(分别占总细胞的1.5% 和7.2%),但在2周龄的膜中却未发现。GOHEL等[31]发现碱性磷酸酶染色阳性细胞始终存在于3-6周的诱导膜中,特别是在4周龄的膜中。同时,诱导膜包含矿化区,茜素红或Von Kossa染色后可见钙化结节形成,主要分布在与PMMA接触的膜内层和中间层[8]。GOHEL等[31]使用RNA测序分析表明,与大鼠骨不连处收集的骨和纤维组织相比,与PMMA相邻的诱导膜和骨都富含成骨细胞的标志基因和通路。然而,GINDRAUX等[39]仅在他们采样的3个人诱导膜中最成熟的一个(14.7个月)中发现了少量的碱性磷酸酶和骨钙素阳性的成骨细胞,该样本的Von Kossa和茜素红染色没有观察到矿化结节。
上述研究报告的膜内是否含成骨细胞结果差异很大的一个原因可能与采样位置有关。多项研究结果表明,诱导膜内存在新骨和新生软骨[6,11-12,22,25-26],这些新生骨组织大多位于骨水泥与骨断端连接处,通常与诱导膜的边缘连续并部分包绕水泥两端[6,11-12,22]。因此,如果膜样本来源于骨缺损的近端或远端,而不是膜中心,理论上可能检测到更多成骨细胞和骨祖细胞。同时,这些研究结果也说明第二阶段手术(植骨)前,诱导膜可能已经开始膜内成骨和软骨内成骨的矿化和整合过程,而植骨后的骨再生开始于截骨端,并由截骨的两端向中心生长,这与TOTH等[6]观察到的结果基本一致。
破骨细胞:关于破骨细胞或破骨前体细胞,即降钙素受体(CTR)、核因子kB受体激活剂(receptor activator of nuclear factor-kB,RANK)或抗酒石酸酸性磷酸酶(TRAP)阳性细胞,是否存在于膜中也不明确。GOURON等[8]在3-6周的大鼠诱导膜上也观察到了抗酒石酸酸性磷酸酶染色阳性细胞,位于膜的边缘与远、近端骨段接触处,以及与PMMA接触的内层细胞区;诱导膜的免疫荧光标记显示,与PMMA接触的细胞层几乎所有细胞都是降钙素受体和RANK阳性细胞。RANK阳性细胞大约占到诱导膜总细胞数的45%-75%。GOHEL等[31]RNA测序分析表明,大鼠的诱导膜富集破骨细胞相关基因。但这些数据还需要进一步证实,如VIATEAU等[22]在绵羊6周的诱导膜中就没有检测到RANK阳性细胞存在。
(4)膜-植骨通讯:少数已发表的研究涉及到诱导膜的膜内成分和自体植骨中的成骨细胞谱系细胞之间的相互作用,这些研究为阐明膜成分可能支持植骨性能的分子机制奠定了基础。PELISSIER等[5]将兔诱导膜蛋白提取物与人骨髓间充质干细胞共培养发现,膜蛋白提取物可以明显促进人骨髓间充质干细胞的增殖和碱性磷酸酶的活性。与该研究相似,WANG等[18]则将兔诱导膜蛋白提取物与小鼠间充质干细胞系C3H10T1/2共培养,发现2-8周的诱导膜在所观察的时间段(9 d)内,也促进了C3H10T1/2的增殖和碱性磷酸酶的表达;该研究还发现,诱导膜促进干细胞增殖和成骨分化的能力与骨膜基本相似。AHO等[25]直接将人的诱导膜与人骨髓来源的间充质干细胞共培养,即使没有成骨诱导剂,膜的存在也增加了间充质干细胞的碱性磷酸酶活性和钙浓度,而添加成骨诱导剂后,两者具有协同促进成骨的作用;研究还发现,4周的诱导膜要比8周的诱导膜体外促进间充质干细胞成骨分化的能力更强。GESSMANN等[29]将不同时间的人诱导膜与间充质干细胞共培养发现,与未采用诱导膜干预的间充质干细胞相比,不同时间的人诱导膜均可增强间充质干细胞的增殖活性,尤其是28 d以上的诱导膜,效果更明显。此外,诱导膜还可以体外促进间充质干细胞表达骨特异性碱性磷酸酶、Ⅰ型前胶原羧基端前肽、骨保护素等成骨相关因子。
上述研究表明,诱导膜内的细胞与植骨区的细胞之间存在着复杂而密切的通讯机制,诱导膜是一个生物结构,膜内细胞可以通过直接或间接的方式促进植骨区成骨相关细胞的分化和矿化,这个过程可能也是诱导膜成功重塑骨缺损的关键原因之一。
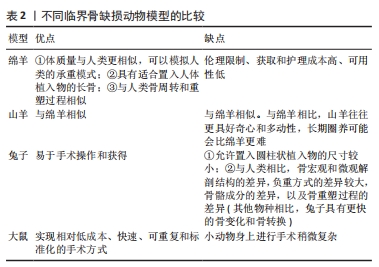
2.4 动物模型的选择 尽管已经构建和描述了许多临界骨缺损动物模型,但对Masquelet诱导膜技术动物模型的关注相对较少。Masquelet在20世纪90年代最先开始在绵羊股骨部位构建了诱导膜模型。随后,陆续有研究在绵羊[7,21-22]、山羊体内建立了诱导膜大型动物模型并进行了相应的干预研究[40],这些大型动物模型已经被很好地鉴定和标准化,并且具有临床相关性。据报道,绵羊的临界骨缺损尺寸为跖骨2.5 cm[7,21-22]、股骨3 cm[4]、胫骨5 cm[20],山羊胫骨的临界骨缺损尺寸也为5 cm[40]。
成年绵羊的优势在于体质量与人类更相似,可以模拟人类的承重模式,并且具有适合置入人体植入物的长骨,这在较小的动物中不能实现[22,41];如果选择跖骨,还可以进行石膏固定,降低植入物失败的风险。绵羊模型还与人类骨周转和重塑过程相似。山羊模型与绵羊模型具有相同的优势,但与绵羊相比山羊往往更具好奇心和多动性,长期圈养可能会比绵羊更难。此外,伦理限制、获取和护理成本高、可用性低是大型动物模型的缺点[22,41]。
兔子因易于手术操作和获得而经常用于骨和植入物研究。已经研究了一些兔子诱导膜模型[42-43]。然而,由于允许置入圆柱状植入物的尺寸较小(直径小于2 mm,长度小于6 mm);与人类相比骨宏观和微观解剖结构的较大差异,负重方式的差异、骨骼成分的差异以及骨重塑过程的差异(与其他物种相比,兔子具有更快的骨变化和骨转换)[41],兔子并不是理想的骨重建模型。
大鼠更常用于研究动物Masquelet技术,大多数是在大鼠股骨中段构建一个平均长度为7 mm(范围0.75-10 mm)骨缺损[44]。尽管在小动物身上进行手术稍微复杂,但在股骨上操作可以提高手术成功率,获得足够的稳定性和抗机械应力,并且可以实现相对低成本、快速、可重复和标准化的手术方式[45]。
除这些因素外,在各物种的诱导膜模型中,植骨的愈合周期也不同,大鼠和兔一般为6-12周取材,绵羊和山羊为12-26周取材[44]。
不同临界骨缺损动物模型的比较见表2。
2.5 骨水泥间隔器的种类和形貌对诱导膜的影响 PMMA骨水泥已被广泛用作诱导膜第一阶段手术的间隔器。由于诱导膜是间隔器与周围组织炎症反应的产物,一些研究试图通过改变间隔器(如材料、表面形貌等)来影响诱导膜形成和骨形成(表3)。

GAIO等[46]将不同粗糙度(1 μm或8 μm)的PMMA或钛植入大鼠股骨缺损区,发现不同粗糙度的PMMA和钛形成的诱导膜膜内Ⅰ型胶原蛋白和弹性蛋白表达量相似,粗糙的表面(8 μm)可以显著提高膜的屈服应变、失效应变和收缩能力等机械性能,而钛诱导的膜显著抑制大于70 kD溶质的跨诱导膜转运。TOTH等[6]继续研究发现,钛诱导的膜比PMMA诱导的膜更厚,粗糙的钛和PMMA(8 μm)会引起炎症因子(白细胞介素6)的适度增加,而光滑的PMMA(1 μm)可以使植骨区骨再生增加,愈合率提高。MCBRIDE-GAGYI等[12]将PMMA、钛和聚乙烯醇间隔器填充于大鼠股骨骨缺损区,PMMA和钛均产生了结构相似的诱导膜,膜内骨形态发生蛋白2、转化生长因子β、血管内皮生长因子和白细胞介素6的表达量相似,只是钛产生的诱导膜比PMMA产生的膜更薄,而聚乙烯醇周围没有明显的膜形成,只有纤维组织和细胞均匀地浸润;植骨10周后发现,PMMA组的骨量多于钛和聚乙烯醇组,聚乙烯醇组的植骨区几乎没有新骨形成,说明PMMA比钛和聚乙烯醇诱导的膜更有利于支持第二阶段的骨再生。LUANGPHAKDY等[40]构建羊5 cm的胫骨诱导膜模型,发现使用纹理化间隔物以增加诱导膜的表面积最终并不会增加新骨形成,但该研究发现刮去诱导膜最内层,即诱导膜的异物反应层,可以促进新骨形成,作者推测膜内血运增多可以促进骨愈合。MA等[42]比较了大鼠股骨缺损处硫酸钙骨水泥和PMMA所诱导出的生物膜,发现两者的组织学特征相似,但硫酸钙诱导的膜产生血管内皮生长因子、骨形态发生蛋白2和转化生长因子β的能力更好,缺损边缘的新骨形成更明显,还可以被完全吸收而不需要二次手术取出,从而认为硫酸钙有可能取代PMMA作为Masquelet技术的新型间隔物。MATHIEU等[47]发现,聚丙烯(1 mL注射器筒)和PMMA在大鼠股骨缺损区形成的诱导膜的结构、细胞密度、骨形态发生蛋白2基本相似;植骨10周后,骨修复区的骨量也相似。DURAND等[48]研究表明,与PMMA相比,偏高岭土间隔器诱导的大鼠股骨诱导膜中,转化生长因子β和骨形态发生蛋白2的水平较高,M1和M2亚型巨噬细胞群更多,骨缺损区的骨痂更丰富,表明偏高岭土比PMMA诱导的膜可更有效地支持骨再生。SILVA JúNIOR等[49]将F18生物玻璃腻子填充于兔子桡骨节段性骨缺损区域,术后21,42 d发现生物材料的不透X射线性和体积逐渐减少,所有动物均形成了层次结构不太明显的诱导膜,主要由纤维胶原组织构成;此外,还观察到正在再生的软骨样和骨样基质、丰富的血管以及由巨噬细胞和多核巨细胞引发的异物反应。ASTUDILLO POTES等[50]发现,3D打印可生物降解的多孔聚己内酯富马酸酯(PCLF)/聚己内酯(PCL)间隔器在大鼠股骨节段性骨缺损区可诱导出和无孔PMMA相似的富含胶原的诱导膜,并且孔隙中有骨形态发生蛋白2表达和编织骨形成,该多孔支架孔隙中的骨化形成表明了其具有骨再生的潜力,可能避免PMMA所需的第二次手术。
上述研究结果表明,PMMA不是唯一可以用作诱导生物膜的材料,可能有更加合适的材料可以诱导出物理、化学和生物特性更佳的生物膜以促进骨形成,筛选其他既成本低廉又能诱导高质量生物膜的材料十分必要,尤其是在资源匮乏、医疗条件有限的环境中。现有的这些材料学的研究仍然是基础研究,其结果也互有矛盾,应用于临床前仍然需要进一步验证。
2.6 骨水泥间隔器所载抗生素对诱导膜的影响 现有的临床研究中,只有PMMA骨水泥作为间隔物的经验。Masquelet不建议使用含有抗生素的PMMA水泥,原因有以下4个[4]:①所载抗生素可能对病菌并无作用,很可能增加致病菌的耐药性;②一些活性抗生素可能会影响和改变诱导膜的生物学特性;③使用非负载抗生素的骨水泥,治疗后无感染的复发,可以作为感染得到控制的指征,避免一过性掩盖感染;④会错误地认为含有抗生素的骨水泥能够治疗骨感染,从而进行不彻底、不充分的清创。然而,据报道大多数临床病例(62.5%)使用了负载抗生素的PMMA,并取得良好的临床效果[51]。目前,尚未有临床研究评估所载抗生素对人体诱导膜的毒性作用。
一些基础研究分析了浸渍抗生素的PMMA对诱导膜形成的影响。最常用于浸渍PMMA的抗生素是庆大霉素、克林霉素、妥布霉素和万古霉素。相对于其他给药途径,这种抗生素给药方式具有在缺损部位的局部浓度更高和全身浓度更低的优势[52-53]。NAU等[32]研究表明,大鼠诱导膜的厚度、弹性纤维的比例、血管化和炎症反应均受到添加到PMMA中的抗生素种类的影响,比如:负载庆大霉素+克林霉素的骨水泥比单纯负载庆大霉素的骨水泥在诱导6周后的诱导膜更薄;与庆大霉素和庆大霉素+
万古霉素相比,庆大霉素+克林霉素骨水泥诱导2周后膜的弹性纤维的比例显著增加;庆大霉素+克林霉素骨水泥诱导的膜内成熟的血管数量在4周时明显高于庆大霉素骨水泥诱导的膜;用庆大霉素诱导的膜中,单核细胞的百分比随着时间的推移(2-6周)趋于增加,而在庆大霉素+克林霉素和庆大霉素+万古霉素组中单核细胞百分比呈降低的趋势。SARITA等[54]研究表明,克林霉素不仅有助于控制局部感染,还可以促进大鼠诱导膜膜内成骨相关因子(骨保护素)的表达。ZIROGLU等[55]研究也表明,掺入了抗生素(庆大霉素、夫西地酸和替考拉宁)的PMMA可通过增加转化生长因子β和血管内皮生长因子等成骨因子的组织水平来改善大鼠股骨诱导膜。DEMIR等[56]发现:无抗生素骨水泥组所诱导的兔股骨诱导膜中的膜质量标志物(血管性血友病因子、白细胞介素6/8、转化生长因子β和血管内皮生长因子等)的表达水平显著高于含庆大霉素和含万古霉素骨水泥组,向骨水泥中添加抗生素对膜有负面影响。CAMPEIRO JUNIOR等[57]也发现,添加头孢唑林的骨水泥不会影响鸡桡骨节段性缺损中诱导膜的厚度,但会对组织学方面产生负面影响,如血管减少、软骨区减少以及炎症持续存在。然而,这些研究未能进一步评估对植骨重塑的影响。
在抗生素的剂量方面,万古霉素是Masquelet中最常用的抗生素,应使用何种剂量仍存在争议。以往研究中,每40 g的PMMA使用万古霉素的剂量为1-10 g。XIE等[58]研究表明,负载不同浓度(1-10 g)万古霉素的PMMA骨水泥(40 g)无明显肝肾毒性;与未添加万古霉素的PMMA相比,负载相对低浓度万古霉素(1-4 g)的PMMA不干扰兔诱导膜的细胞增殖、成骨和成血管能力,甚至可以提高这些能力;相反,负载相对高浓度万古霉素(6-10 g)的PMMA对膜内细胞增殖、成骨和成血管能力均有负面影响。他们接下来的研究发现,无论是影像学还是组织学结果,较低浓度万古霉素(0-4 g)组均要比较高浓度万古霉素(6,8,10 g)组的新骨形成更多、愈合速度更快[59],因此,作者认为,大剂量万古霉素可能会损害间充质干细胞的活性和成骨分化。
目前,是否负载抗生素主要取决于临床医生的偏好,因为清除感染对于手术成功非常重要,PMMA经常经验性负载抗生素以增加清除感染的可能性。现有的临床观察暂未报道抗生素对诱导膜有负面影响,而基础研究关于抗生素对诱导膜形成和性质影响的结论并不一致。此外,抗生素的剂量(主要是万古霉素)推荐每40 g PMMA负载1-4 g抗生素。但是正如Masquelet提倡的[4]:彻底清创仍然是解决感染的关键,不能过分依赖局部抗生素的应用。
2.7 固定方式的选择 正如GIANNOUDIS等[60]所报道的,机械稳定性是骨愈合的关键因素之一。MASQUELET[61]认为,外固定器、钢板和髓内钉都可以选用,但第二阶段手术之后,早期应尽可能刚性固定以促进植骨的血运重建,随后弹性固定以增强植骨的皮质化。Masquelet不建议使用提供刚性稳定性的锁定螺钉,它可能成为植骨皮质化的障碍。坚强固定在早期需要,但在后期则是有害的。
不同的固定方式在实验动物身上进行过验证。TOTH等[6]使用单侧外固定器在大鼠股骨重现了Masquelet技术,他们发现一期术后,固定针松动的发生率为4.8%(2/42),但二期术后,当提供机械支持的间隔器不再存在时,松动发生率为26%(15/58)。MATHIEU等[47]采用微型外固定器(RatExFix; RISystem)固定于大鼠诱导膜模型的股骨6 mm缺损部位,该固定器因靠近骨干部位,在皮肤缝合后可埋于皮下而不外露,植骨后发现22%(11/50)的大鼠出现固定失败。XIE等[58-59]采用克氏针固定兔桡骨部位10 mm的骨缺损,一期术后不同时间点(2,4,6周)均检测到结构分明且具有成血管、成骨能力的诱导膜;植骨8周后,骨缺损修复。GRUBER等[11]使用髓内固定装置-AO Locking Rat Nail(AO Davos,瑞士)在大鼠股骨中段对5 mm的缺损进行了固定,固定4,8,16周分别进行影像学和组织学检测,发现这种锁定髓内钉固定可靠,大鼠耐受性良好并且诱导出了良好的生物膜。然而,该研究未进行第二阶段手术,从而进一步对锁定髓内钉固定下植骨的愈合情况进行评估。目前,大多数文献报道的动物诱导膜模型仍然以钢板固定为主。SUN等[44]纳入了47项研究进行系统评价发现,62%(29/47)的研究使用钢板和螺钉,11%(5/47)使用髓内钉,9%(4/47)使用外固定架,2%(1/47)使用克氏针。
外固定具有固定灵活、操作简便、对诱导膜和周围软组织的影响小的优点,缺点主要是固定的稳定性较差,针孔感染的风险较高[45],同时,由于动物无依从性,术后术侧肢体不能制动,并且会互相撕咬外固定装置,所以固定失效、骨端移位的风险较高。相对于外固定器,钢板和螺钉更加轻便、稳定性更高,操作可能比外固定器更简单,复位效果更好;缺点包括容易损伤骨膜、存在弯曲或折断的风险;此外,钢板会诱导出自己的膜,诱导膜取材时,会受到钢板膜的干扰,使取材难度加大[45]。相比之下,髓内固定装置除与钢板具有相同的优点外[45],还可以最大限度地减少固定材料对诱导膜形成的干扰,但在扩髓时易发生骨折,且该设备价格昂贵[11,26,35]。总之,固定的稳定性取决于所涉及的骨缺损的部位和大小,固定方法的选择必须实用、可重复、标准化且成本低。
2.8 骨组织工程材料 获得足够的高质量自体松质骨是诱导膜技术成功的一项重要的临床障碍。因此,已有多项研究测试了可以替代植骨的生物材料或促进植骨矿化的生长因子。
2.8.1 生长因子 在动物模型中,重组人骨形态发生蛋白7(rhBMP-7)和重组人骨形态发生蛋白2(rhBMP-2)已被证明可有效改善骨愈合[62-63]。受此启发,Masquelet等将重组人骨形态发生蛋白7添加到自体骨以增强皮质骨的快速形成。Masquelet等[4]在2000-2004年间治疗了11例感染性骨不连患者,平均缺损10.5 cm(5-18 cm);在第二阶段,将自体松质骨与3.5 mg的重组人骨形态发生蛋白7混合使用,尽管10例患者在平均11.5个月(6-18个月)实现骨愈合,但作者观察到:所有患者在植骨2个月后,植骨区域均出现局限性骨吸收灶。作者认为,膜的存在使骨形态发生蛋白不能在周围软组织中扩散,导致植骨中骨形态发生蛋白浓度过高,可能对成骨细胞分化有相反的作用,导致植骨部分吸收。因而,不确定生长因子的效果。
MI等[64]一项系统评价分析了多项临床研究结果发现,第二阶段手术时采用骨形态发生蛋白辅助植骨,骨形态发生蛋白治疗组的愈合率为97.22%,而未使用骨形态发生蛋白治疗组的愈合率为91.8%,总体来看,添加生长因子组的愈合率要略高于未添加任何生长因子组的愈合率。
其他生长因子也有报道。YILMAZ等[43]研究了浓缩生长因子(CGF)对兔诱导膜质量的影响,结果发现与对照组相比,浓缩生长因子可以提高早期(3周)和晚期(6周)诱导膜的厚度、弹性纤维的比例、炎症反应、细胞增殖、血管和干细胞的数量。然而,该研究未能进一步探讨浓缩生长因子干预下的诱导膜对植骨矿化的影响。BILAL等[65]研究了富血小板血浆或表皮生长因子(EGF)对大鼠Maqquelet技术的影响,第一阶段手术后6周,富血小板血浆组和表皮生长因子组的膜中血小板内皮细胞黏附分子(CD31)、转化生长因子和血管内皮生长因子水平显著高于未使用生长因子的对照组;第二阶段手术后6周,骨内血管内皮生长因子和骨形态发生蛋白2的水平也显著高于对照组,说明富血小板血浆和表皮生长因子有可能用作Masquelet技术中加速大段骨缺损愈合的辅助手段。
2.8.2 骨修复材料 生物材料大体可分为4类[66]:天然聚合物,如胶原蛋白、纤维蛋白和壳聚糖;合成聚合物,如聚乳酸(PLA)、聚己内酯(PCL)和羟基乙酸共聚物(PLGA);陶瓷,如羟基磷灰石、磷酸钙和生物活性玻璃;以及复合材料。这些材料因为具有生物相容性、骨传导性和可降解性等优势,已被应用于基础和临床研究中。
VIATEAU等[7]使用搭载间充质干细胞的天然珊瑚支架(含99%碳酸钙和1%有机材料)填充绵羊模型的诱导膜内时发现,与植入自体骨相比,6个月时两者的缺损区骨量相似,但自体骨组的X射线评分明显更高(21% vs.100%的愈合皮质);如果单独使用天然珊瑚支架,缺损区几乎没有新骨形成。该研究证明:间充质干细胞/天然珊瑚支架可用于修复大段骨缺损,并且它们的成骨能力接近自体骨;然而,需要优化许多重要参数,例如支架再吸收率和支架上播种间充质干细胞的数量。
NAU等[67]在大鼠诱导膜内植入负载不同细胞的β-磷酸三钙支架进行了比较,分别为β-磷酸三钙支架、同源骨、β-磷酸三钙支架+骨髓内皮祖细胞/脾来源的间充质干细胞和β-磷酸三钙支架+骨髓单个核细胞。植入8周后发现,各组在生物力学稳定性、新骨形成(骨钙素含量)、骨矿物质密度和血管密度方面,无统计学差异;但从趋势来看,同源骨最优,髓内皮祖细胞/间充质干细胞和骨髓单个核细胞次之,单独β-磷酸三钙支架最差。该研究表明了在β-磷酸三钙上种植再生细胞(如骨髓单个核细胞或骨髓内皮祖细胞联合间充质干细胞)以支持诱导膜骨愈合的可行性,其程度与同源骨相似。
其他来源的干细胞也可能有作用。DILOGO等[68]报道了1例采用改良Masquelet 技术治疗的右侧股骨感染性骨不连患者,诱导膜第二阶段的手术中,在12 cm的缺损区域填充了脐带间充质干细胞、骨形态发生蛋白2和羟基磷灰石,术后6个月实现了临床愈合,11个月可以完全负重。这是首例将Masquelet技术与脐带间充质干细胞、骨形态发生蛋白2和羟基磷灰石联合使用的临床病例。
与上述研究结果不同,DEBAUN等[69]在大鼠诱导膜模型中插入3D打印的聚己内酯/β-磷酸三钙支架,与单独使用支架(无诱导膜)和聚己内酯人工合成膜联合支架相比,诱导膜联合聚己内酯/β-磷酸三钙支架组的骨愈合评分明显更高和再生骨量明显更大,证明该方法可以支持诱导膜内的骨形成。
BOSEMARK等[70]在使用环氧树脂间隔器获得诱导膜的大鼠模型中,研究了合成支架(含80% 磷酸三钙和20%羟基磷灰石)单独使用或与骨形态发生蛋白7和双膦酸盐联合使用是否可以促进骨愈合,X射线结果发现:单独使用支架骨缺损没有愈合;单独使用骨形态发生蛋白7有70%(7/10)的缺损可愈合,在愈合的股骨中,有2个在近端或远端有可见的骨折线;骨形态发生蛋白7+支架有100%(10/10)的缺损可愈合,但90%(9/10)的样本在近端和/或远端仍有可见的骨折线;骨形态发生蛋白7+支架+
双膦酸盐有90%(9/10)的缺损可愈合,9个愈合样本中只有1个有可见的骨折线。作者认为,骨形态发生蛋白可诱导成骨细胞的分化和增殖,但也会诱导破骨细胞活化,骨形态发生蛋白介导的破骨细胞活化可以通过给予双膦酸盐抵消,骨形态发生蛋白7应用2周后全身使用双膦酸盐可使骨形态发生蛋白7的骨愈合能力翻倍。
LEIBLEIN等[71]评估了植骨替代物Herafill?的颗粒大小对Masquelet技术大鼠模型的影响,结果表明与较大直径(3 mm)的颗粒组相比,较小(0.5-1.0 mm)颗粒组在8周后骨缺损区的骨痂明显更多,骨密度明显更高,巨噬细胞的聚集增加,但这并没有导致缺损区的生物力学稳定性增加。原因可能在于:①骨痂组织主要围绕缺损内的单个颗粒以岛状区域的形式形成,8周后还未融合;②与β-磷酸三钙相比,Herafill?颗粒的硬度较低。此外,他们将颗粒与骨诱导剂(骨髓来源的单核细胞)结合使用,发现并没有取得更好的愈合效果。接下来的研究中,LEIBLEIN等[72]评估了Herafill?的颗粒大小对Masquelet技术大鼠模型缺损区周围骨膜再生的影响,发现与较大直径(3 mm)的颗粒组相比,较小(0.5-1.0 mm)颗粒组在8周后骨膜的再生、发育显著增加;同时,在用小颗粒处理的骨缺损中,与诱导膜直接相邻处的新骨形成明显增加。作者对这一结果的解释是,小颗粒的包装更紧密,增加了与缺损边缘处骨皮质的接触,从而引导骨膜结构可以沿着它生长;此外,骨膜与诱导膜融合的增加,这可能有利于骨再生。
骨组织工程材料的应用研究汇总,见表4。

上述的研究结果表明,单独植入生物支架时大多数的骨缺损均愈合不理想,而在大部分研究中使用生物添加剂(细胞或生长因子)提高了骨愈合率,甚至取得了与同源骨相似的愈合能力。然而,当前此类研究还相对较少,且这些材料的构成和降解率、细胞和生长因子的加载浓度以及体内结果具有高度可变性,因此,难以就替代移植材料及其负载生物添加剂的效果得出明确的结论,但将组织工程应用于诱导膜的骨重建中仍然是一种很有前景的方法。

| [1] KUMAWAT V, BANDYOPADHYAY-GHOSH S, GHOSH S. An overview of translational research in bone graft biomaterials. Journal of biomaterials science. Polymer edition. 2023;34(4):497-540. [2] AUREGAN JC, BEGUE T. Induced membrane for treatment of critical sized bone defect: a review of experimental and clinical experiences. Int Orthop. 2014;38(9):1971-1978. [3] MASQUELET A C, FITOUSSI F, BEGUE T, et al. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45(3):346-353. [4] MASQUELET A C, BEGUE T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41(1) 27-37. [5] PELISSIER P, MASQUELET AC, BAREILLE R, et al. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22(1):73-79. [6] TOTH Z, ROI M, EVANS E, et al. Masquelet Technique: Effects of Spacer Material and Micro-topography on Factor Expression and Bone Regeneration. Ann Biomed Eng. 2019;47(1):174-189. [7] VIATEAU V, GUILLEMIN G, BOUSSON V, et al. Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25(6):741-749. [8] GOURON R, PETIT L, BOUDOT C, et al. Osteoclasts and their precursors are present in the induced-membrane during bone reconstruction using the Masquelet technique. J Tissue Eng Regen Med. 2017;11(2):382-389. [9] ALFORD AI, NICOLAOU D, HAKE M, et al. Masquelet’s induced membrane technique: Review of current concepts and future directions. J Orthop Res. 2021;39(4):707-718. [10] HENRICH D, SEEBACH C, NAU C, et al. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J Tissue Eng Regen Med. 2016;10(10):E382-E396. [11] GRUBER H, GETTYS F, MONTIJO H, et al. Genomewide molecular and biologic characterization of biomembrane formation adjacent to a methacrylate spacer in the rat femoral segmental defect model. J Orthop Trauma. 2013;27(5):290-297. [12] MCBRIDE-GAGYI S, TOTH Z, KIM D, et al. Altering spacer material affects bone regeneration in the Masquelet technique in a rat femoral defect. J Orthop Res. 2018;36:2228-2238. [13] LIU H, HU G, SHANG P, et al. Histological characteristics of induced membranes in subcutaneous, intramuscular sites and bone defect. Orthop Trauma Surg Res. 2013;99(8):959-964. [14] CUTHBERT R J, CHURCHMAN S M, TAN H B, et al. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone. 2013;57(2):484-492. [15] JOYCE ME, ROBERTS AB, SPORN MB, et al. Transforming growth-factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. Clin Res. 1990;38(2):A407-A407. [16] PHILLIPS A. Overview of the fracture healing cascade. Injury. 2005:36 Suppl 3:S5-7. [17] CHEN G, DENG C, LI Y. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272-288. [18] WANG X, WEI F, LUO F, et al. Induction of granulation tissue for the secretion of growth factors and the promotion of bone defect repair. J Orthop Surg Res. 2015;10:1-8. [19] HSIEH M, WU C, CHEN C, et al. BMP-2 gene transfection of bone marrow stromal cells to induce osteoblastic differentiation in a rat calvarial defect model. Mater Sci Eng C Mater Biol Appl. 2018:91:806-816. [20] CHRISTOU C, OLIVER R, YU Y, et al. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PloS one. 2014;9(12):e114122. [21] VIATEAU V, BENSIDHOUM M, GUILLEMIN G, et al. Use of the induced membrane technique for bone tissue engineering purposes: animal studies. Orthop Clin North Am. 2010;41(1):49-56. [22] VIATEAU V, GUILLEMIN G, CALANDO Y, et al. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: an ovine model. Vet Surg. 2006;35(5):445-452. [23] TANG Q, TONG M, ZHENG G, et al. Masquelet’s induced membrane promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by activating the Smad and MAPK pathways. Am J Transl Res. 2018;10(4):1211-1219. [24] WU M, CHEN G, LI Y. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. [25] AHO OM, LEHENKARI P, RISTINIEMI J, et al. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am. 2013;95(7): 597-604. [26] GRUBER H E, ODE G, HOELSCHER G, et al. Osteogenic, stem cell and molecular characterisation of the human induced membrane from extremity bone defects. Bone Joint Res. 2016;5(4):106-115. [27] WANG W, YEUNG K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2(4):224-247. [28] TANG Q, JIN H, TONG M, et al. Inhibition of Dll4/Notch1 pathway promotes angiogenesis of Masquelet’s induced membrane in rats. Exp Mol Med. 2018;50(4):1-15. [29] GESSMANN J, ROSTEIUS T, BAECKER H, et al. Is the bioactivity of induced membranes time dependent?. Eur J Trauma Emerg Surg. 2022;48(4):3051-3061. [30] DURAND M, BARBIER L, MATHIEU L, et al. Towards Understanding Therapeutic Failures in Masquelet Surgery: First Evidence that Defective Induced Membrane Properties are Associated with Clinical Failures. J Clin Med. 2020;9(2):450. [31] GOHEL N, SENOS R, GOLDSTEIN S, et al. Evaluation of global gene expression in regenerate tissues during Masquelet treatment. J Orthop Res. 2020;38(10):2120-2130. [32] NAU C, SEEBACH C, TRUMM A, et al. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury. 2016;47(2):325-334. [33] BATOON L, MILLARD S, RAGGATT L, et al. Osteomacs and Bone Regeneration. Curr Osteoporos Rep. 2017;15(4):385-395. [34] WANG W, ZUO R, LONG H, et al. Advances in the Masquelet technique: Myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. 2020;108:223-236. [35] GRUBER HE, RILEY FE, HOELSCHER GL, et al. Osteogenic and chondrogenic potential of biomembrane cells from the PMMA-segmental defect rat model. J Orthop Res. 2012;30(8):1198-1212. [36] PONTE A, MARAIS E, GALLAY N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737-1745. [37] MAKSYM R, TARNOWSKI M, GRYMULA K, et al. The role of stromal-derived factor-1--CXCR7 axis in development and cancer. Eur J Pharmacol. 2009;625(1-3):31-40. [38] MILLER R, BANISADR G, BHATTACHARYYA B. CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol. 2008; 198(1-2):31-38. [39] GINDRAUX F, LOISEL F, BOURGEOIS M, et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur J Trauma Emerg Surg. 2020;46(2):301-312. [40] LUANGPHAKDY V, ELIZABETH PLUHAR G, PIUZZI N S, et al. The Effect of Surgical Technique and Spacer Texture on Bone Regeneration: A Caprine Study Using the Masquelet Technique. Clin Orthop Relat Res. 2017;475(10): 2575-2585. [41] PEARCE A, RICHARDS R, MILZ S, et al. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007:13:1-10. [42] MA Y, JIANG N, ZHANG X, et al. Calcium sulfate induced versus PMMA-induced membrane in a critical-sized femoral defect in a rat model. Sci Rep. 2018;8(1):637. [43] YILMAZ O, OZMERIC A, ALEMDAROGLU KB, et al. Effects of concentrated growth factors (CGF) on the quality of the induced membrane in Masquelet’s technique - An experimental study in rabbits. Injury. 2018;49(8):1497-1503. [44] SUN H, GODBOUT C, HALI K, et al. The induced membrane technique in animal models: a systematic review. OTA Int. 2022;5(1 Suppl):e176. [45] KLEIN C, MONET M, BARBIER V, et al. The Masquelet technique: Current concepts, animal models, and perspectives. J Tissue Eng Regen Med. 2020;14(9):1349-1359. [46] GAIO N, MARTINO A, TOTH Z, et al. Masquelet technique: The effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J Biomech. 2018; 72:53-62. [47] MATHIEU L, MURISON J, DE ROUSIERS A, et al. The Masquelet Technique: Can Disposable Polypropylene Syringes be an Alternative to Standard PMMA Spacers? A Rat Bone Defect Model. Clin Orthop Relat Res. 2021; 479(12):2737-2751. [48] DURAND M, OGER M, NIKOVICS K, et al. Influence of the Immune Microenvironment Provided by Implanted Biomaterials on the Biological Properties of Masquelet-Induced Membranes in Rats: Metakaolin as an Alternative Spacer. Biomedicines. 2022;10(12):3017. [49] SILVA JÚNIOR JIS, RAHAL SC, CORIS JGF, et al. Use of F18 bioglass putty for induced membrane technique in segmental bone defect of the radius in rabbits. Acta Cir Bras. 2024;39:e392424. [50] ASTUDILLO POTES MD, MITRA I, HANSON K, et al. Biodegradable poly(caprolactone fumarate) 3D printed scaffolds for segmental bone defects within the Masquelet technique. J Orthop Res. 2024;24:1-10. [51] MORELLI I, DRAGO L, GEORGE DA, et al. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016;47:S68-S76. [52] KENT ME, RAPP RP, SMITH KM. Antibiotic beads and osteomyelitis: Here today, what’s coming tomorrow? Orthopedics. 2006;29(7):599-603. [53] NAAL F, SALZMANN G, VON KNOCH F, et al. The effects of clindamycin on human osteoblasts in vitro. Arch Orthop Trauma Surg. 2008;128(3):317-323. [54] SHAH S, SMITH B, TATARA A, et al. Effects of Local Antibiotic Delivery from Porous Space Maintainers on Infection Clearance and Induction of an Osteogenic Membrane in an Infected Bone Defect. Tissue Eng. Part A. 2017;23(3-4):91-100. [55] ZIROGLU N, KOLUMAN A, KALECI B, et al. The antibiotics supplemented bone cement improved the masquelet’s induced membrane in a rat femur critical size defect model. Injury. 2023;54(2):329-338. [56] DEMIR M, GUNAY MC, ADIGUZEL IF, et al. Does the use of antibiotic spacer disrupt induced membrane function? Injury. 2023;54(4):1055-1064. [57] CAMPEIRO JUNIOR LD, RAHAL SC, SOUZA MA, et al. Induced membrane technique using bone cement with or without cefazolin in chicken segmental radius defect. Front Vet Sci. 2023;10:1027951. [58] XIE J, WANG W, FAN X, et al. Masquelet technique: Effects of vancomycin concentration on quality of the induced membrane. Injury. 2022;53(3):868-877. [59] XIE J, WANG W, FAN X, et al. Effects of PMMA spacer loaded with varying vancomycin concentrations on bone regeneration in the Masquelet technique. Sci Rep. 2022;12(1):4255. [60] GIANNOUDIS P, EINHORN T, MARSH D. Fracture healing: the diamond concept. Injury. 2007:38 Suppl 4:S3-6. [61] MASQUELET AC. Induced Membrane Technique: Pearls and Pitfalls. J Orthop Trauma. 2017;31:S36-S38. [62] YASKO A, LANE J, FELLINGER E, et al. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74(5):659-670. [63] COOK S, BAFFES G, WOLFE M, et al. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop Relat Res. 1994:(301):302-312. [64] MI M, PAPAKOSTIDIS C, WU X, et al. Mixed results with the Masquelet technique: A fact or a myth? Injury. 2020;51(2):132-135. [65] BILAL O, TOPAK D, KINAS M, et al. Epidermal growth factor or platelet-rich plasma combined with induced membrane technique in the treatment of segmental femur defects: an experimental study. J Orthop Surg Res. 2020;15(1):601. [66] INZANA J, SCHWARZ E, KATES S, et al. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials. 2016;81:58-71. [67] NAU C, SIMON S, SCHAIBLE A, et al. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat’s femur. Injury. 2018;49(10):1721-1731. [68] DILOGO IH, PRIMAPUTRA MRA, PAWITAN JA, et al. Modified Masquelet technique using allogeneic umbilical cord-derived mesenchymal stem cells for infected non-union femoral shaft fracture with a 12 cm bone defect: A case report. Int J Surg Case Rep. 2017;34:11-16. [69] DEBAUN MR, STAHL AM, DAOUD AI, et al. Preclinical induced membrane model to evaluate synthetic implants for healing critical bone defects without autograft. J Orthop Res. 2019;37(1):60-68. [70] BOSEMARK P, PERDIKOURI C, PELKONEN M, et al. The Masquelet Induced Membrane Technique With BMP and a Synthetic Scaffold Can Heal a Rat Femoral Critical Size Defect. J Orthop Res. 2015;33(4):488-495. [71] LEIBLEIN M, KOCH E, WINKENBACH A, et al. Size matters: Effect of granule size of the bone graft substitute (Herafill(R)) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J Biomed Mater Res B Appl Biomater. 2020;108(4):1469-1482. [72] LEIBLEIN M, WINKENBACH A, KOCH E, et al. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: a study in rat femur critical size bone defect model. Eur J Trauma Emerg Surg. 2022;48(1):679-687. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 韩海慧, 孟晓辉, 徐 博, 冉 磊, 施 杞, 肖涟波. 成纤维细胞生长因子受体1抑制剂对胶原诱导关节炎模型大鼠骨破坏的影响[J]. 中国组织工程研究, 2025, 29(5): 968-977. |
| [3] | 李 帅, 刘 桦, 商永慧, 刘义琮, 赵启航, 刘 文. 安氏Ⅱ类错牙合畸形佩戴Twin-block矫治器时上颌骨的应力分布[J]. 中国组织工程研究, 2025, 29(5): 881-887. |
| [4] | 张立创, 杨 雯, 丁广江, 李培坤, 肖忠宇, 陈 营, 方 雪, 张 腾. 个体化单侧椎弓根外入路与双侧椎弓根入路椎体成形后骨水泥的弥散效果[J]. 中国组织工程研究, 2025, 29(4): 800-808. |
| [5] | 赵红霞, 孙政伟, 韩 阳, 吴学超, 韩 静. 富血小板纤维蛋白复合甲基丙烯酰化明胶水凝胶的促成骨性能[J]. 中国组织工程研究, 2025, 29(4): 809-817. |
| [6] | 肖 放, 黄 雷, 王 琳. 磁性纳米材料与磁场效应加速骨损伤修复[J]. 中国组织工程研究, 2025, 29(4): 827-838. |
| [7] | 刘浩洋, 谢 强, 沈梦然, 任岩松, 马金辉, 王佰亮, 岳德波, 王卫国. 可降解锌基合金在骨缺损修复重建中的应用及研究热点和不足[J]. 中国组织工程研究, 2025, 29(4): 839-845. |
| [8] | 李明哲, 叶翔凌, 王 冰, 余 翔. 负载纳米钽的液晶显示光固化聚乳酸支架制备及促成骨性能[J]. 中国组织工程研究, 2025, 29(4): 670-677. |
| [9] | 周 洋, 刘可鑫, 王得利, 孙 璋. 工程化细胞外囊泡修复骨缺损的再生作用[J]. 中国组织工程研究, 2025, 29(36): 7839-7847. |
| [10] | 王景帅, 张晓彤, 张彦歌, 万泽东, 孔令伟, 曹海营, 金 宇. Mg-Li-Gd合金与不锈钢髓内针植入固定大鼠股骨环形半缺损的比较[J]. 中国组织工程研究, 2025, 29(34): 7261-7268. |
| [11] | 任 波, 唐永亮, 李 妮, 刘邦定. 温敏抗菌水凝胶治疗感染性骨缺损[J]. 中国组织工程研究, 2025, 29(34): 7269-7277. |
| [12] | 盛文博, 刘丙立, 李四波, 敖荣广, 禹宝庆. 椎体骨水泥强化联合短节段经皮骨水泥螺钉内固定治疗Ⅱ期Kümmell病[J]. 中国组织工程研究, 2025, 29(34): 7286-7292. |
| [13] | 张晓宇, 韦善文, 方佳炜, 倪 莉. 普鲁士蓝纳米粒子抗氧化恢复退变髓核细胞线粒体功能[J]. 中国组织工程研究, 2025, 29(34): 7318-7325. |
| [14] | 吴子炜, 罗诒财, 韦银格, 廖红兵. 聚乳酸-羟基乙酸共聚物在口腔医学领域的应用[J]. 中国组织工程研究, 2025, 29(34): 7393-7404. |
| [15] | 邓光慧, 向 炜, 苏其帆, 陈小鱼, 王亮为, 万志宏, 吴佳奇, 陈孝均. 骨质疏松性股骨髁骨缺损兔模型制备及其临界值[J]. 中国组织工程研究, 2025, 29(30): 6426-6433. |
1.1.1 检索人及检索时间 第一作者在2024年5月进行检索。
1.1.2 检索文献时限 1986年1月至2024年5月。
1.1.3 检索数据库 PubMed、Web of Science数据库和中国知网(CNKI)数据库。
1.1.4 检索词 英文检索词为“masquelet technique,induced membrane technique”;中文检索词为“masquelet技术,诱导膜技术”。
1.1.5 检索文献类型 实验类研究原著、综述、荟萃分析等。
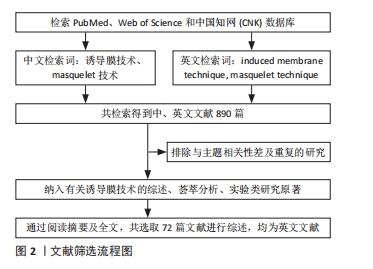
1.1.6 检索策略 中英文数据库检索策略,见图1。
1.2 资料的纳入与排除标准
纳入标准:①与诱导膜技术相关的实验类文章;②论述诱导膜技术相关的综述、荟萃分析类文章。
排除标准:与主题相关性差和重复性研究。
1.3 资料提取与文献质量评估
资料提取:研究文献由相互独立的3人提取并通过小组多次讨论解决分歧。信息记录侧重诱导膜技术实验类的研究进展。
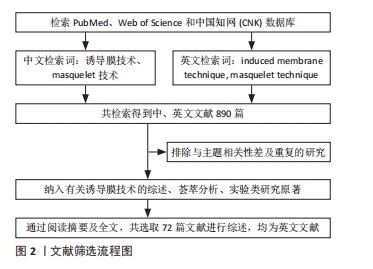
文献质量评估:通过上述计算机检索与手工检索,共检索到890篇参考文献。按入选标准进行人工筛选,排除与主题相关性差和重复的文献,最终纳入72篇文献。
文献筛选流程见图2。
尽管诱导膜技术已经取得了一定的研究进展和临床应用经验,但其技术成熟度仍有待提高,目前尚缺乏统一的技术标准和操作规范来指导临床应用。其次,诱导膜技术成骨的具体机制尚未完全明确,从而限制了该技术的进一步发展和优化。此外,目前用于诱导膜技术的生物材料在性能上仍存在一些局限性,如生物相容性、降解速度、机械强度等方面的不足,这些不足可能影响诱导膜的形成和稳定性,进而影响治疗效果。最后,目前关于诱导膜技术的临床研究的综述较多,基础研究的综述相对较少。
3.2 作者综述区别于他人他篇的特点 此文以诱导膜技术的基础研究为切入点,尽可能覆盖它的产生背景、修复机制和优势、膜的特征、膜-植骨通讯、动物模型的选择、骨水泥的种类、所载抗生素对诱导膜的影响、固定方式的选择和骨组织工程材料等方面的主要基础研究成果,并深入剖析了关键发现和趋势。在总结现有研究的同时,也对研究的方法、结论等进行批判性分析,指出存在的局限性和未来的研究方向。
3.3 综述的局限性 首先,文章主要综述了诱导膜技术的基础研究结果,这些基础研究依赖于动物模型,动物模型在生理、病理及药物反应等方面与人类存在显著差异,种属差异可能导致研究结果在人体中的适用性受限。其次,基础研究的一个显著局限性在于研究结果的不一致性。不同实验室间的差异,包括实验条件、操作细节、实验材料、动物种类等的微小变化,都可能导致研究结果出现偏差,从而影响结果的准确性和一致性。最后,该文涵盖的方向较多,在文献选择和解读过程中,可能受到个人兴趣、研究背景、检索策略等主观因素的影响,导致部分相关文献被遗漏或过度强调,造成综述结果的偏倚。
3.4 综述的重要意义 文章系统地梳理了诱导膜技术在临界尺寸骨缺损修复领域的应用现状与基础研究成果。根据目前的文献研究结果,可以得出以下结论:①Masquelet诱导膜技术是实现临界尺寸骨缺损修复的有效手段,其修复骨缺损的机制尚不明确,但可以确定的是,膜和植骨两者缺一不可。②诱导膜是一种富含多种骨形成相关细胞、生长因子和血管的分层较明显的组织,其血管化和生长因子的分泌随时间而动态变化,但两阶段手术的间隔周期不影响最终的愈合效果。③对于诱导膜动物模型选择而言,每个物种有各自的优点和缺点。在已经报道的模型中,从解剖结构、负重模式和骨重塑的相似度来讲,羊更为接近;从饲养成本和难度、造模周期来讲,大鼠更合适。④PMMA不是唯一可以用做诱导生物膜的材料,可能有更加合适的材料可以诱导出更高质量的诱导膜。同时,骨水泥表面形貌的改变会干扰诱导膜的物理、化学或生物学特性,但是否会影响后期植骨矿化进程还不明确。⑤骨水泥是否负载抗生素主要取决于外科医生的偏好,大多数的临床研究中会经验性负载抗生素,剂量为每40 g PMMA负载抗生素1-10 g,该剂量抗生素的使用未影响到愈合效果。基础研究推荐的抗生素的剂量(主要是万古霉素)为每40 g的PMMA骨水泥负载1-4 g万古霉素,超过该剂量可能会对膜成骨和成血管能力产生负面影响。⑥对于动物(特别是大鼠)的固定方式而言,根据文献报道的使用数量,钢板使用更为广泛,固定方式更加可靠,可重复性更高。⑦在诱导膜技术的第二阶段,仍然没有理想的生物支架可以替代自体骨。随着组织工程的技术进步(例如,新的支架、干细胞和生长因子制备),可能会产生新的材料和方法促进Masquelet技术的诱导膜形成和骨修复能力。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:
临界尺寸骨缺损:是指缺损长度超过一定尺寸后,无法自行愈合的骨缺损。目前,“临界”的精确尺寸或体积尚缺乏共识。无论是人还是动物模型,影响骨缺损修复的因素有很多,如:是否存在骨周向性丢失、解剖位置(骨干/干骺端/关节)、软组织条件(包括骨膜损伤)、感染、年龄、损伤机制,以及是否存在慢性疾病和其他合并症等。总之,关于临界尺寸骨缺损的定义仍然需要进一步分类、细化和证实。诱导膜技术:也称Masquelet技术,是一种重建临界尺寸骨缺损的新技术,由法国Masquelet在1986年首先发现并开始应用于临床。该技术主要包括2个步骤:第一期,彻底清除炎症所涉及的骨段,并在残留的骨缺损区域植入聚甲基丙烯酸甲酯(PMMA)骨水泥占位器,来诱导生物膜的形成;第二期,一期术后6-8周,PMMA骨水泥的周围可见诱导膜形成,此时沿原术口切开诱导膜,去除PMMA骨水泥占位器,并将自体或异体骨植入诱导膜中,等待骨缺损愈合。
尽管诱导膜技术已经取得了一定的研究进展和临床应用经验,但其技术成熟度仍有待提高。目前尚缺乏统一的技术标准和操作规范来指导临床应用。其次,诱导膜技术成骨的具体机制尚未完全明确,从而限制了该技术的进一步发展和优化。此外,目前用于诱导膜技术的生物材料在性能上仍存在一些局限性,如生物相容性、降解速度、机械强度等方面的不足。这些不足可能影响诱导膜的形成和稳定性,进而影响治疗效果。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||