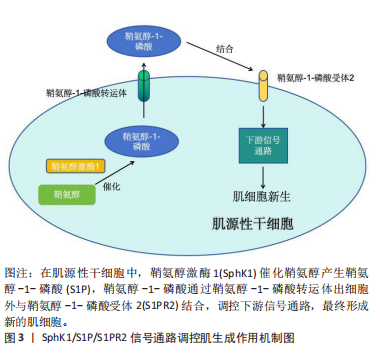
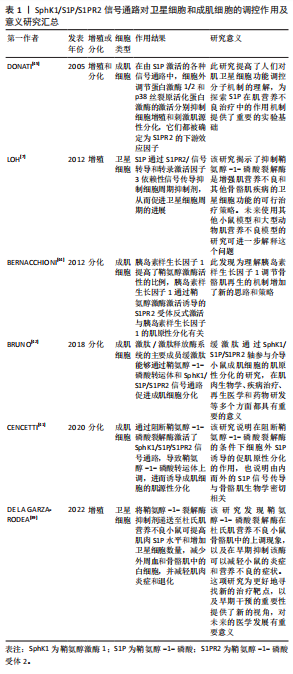
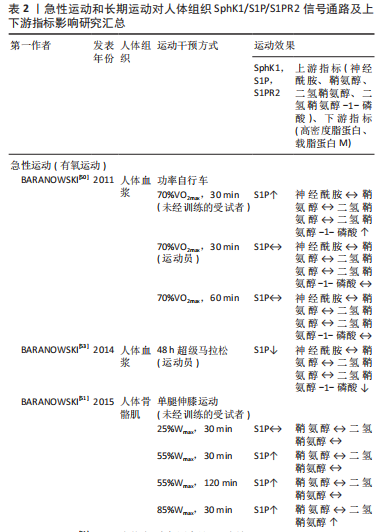
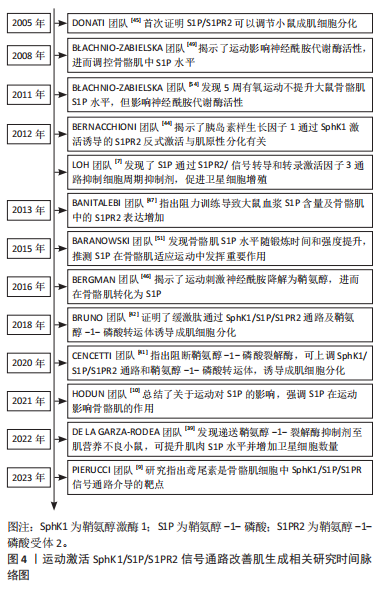
[1] BOYER JG, HUO J, HAN S, et al. Depletion of skeletal muscle satellite cells attenuates pathology in muscular dystrophy. Nat Commun. 2022; 13(1):2940.
[2] 王震,蔺海旗,何霏,等.运动激活骨骼肌卫星细胞:增龄性肌衰减症及肌肉损伤修复的运动预防和治疗[J].中国组织工程研究, 2021,25(23):3752-3759.
[3] 赵婷,郭欣雨,郑雨林,等.雌激素对骨骼肌成肌细胞增殖、分化及迁移的影响[J].中国老年学杂志,2022,42(14):3554-3558.
[4] 王素素,李丽凤,张一民.运动干预老年人肌少症近10年研究进展及国际热点可视化分析[J].中国组织工程研究,2022,26(14): 2223-2230.
[5] 王燕,肖雄,郭峰,等.和血柔肝方对肝纤维化大鼠SphK1/S1P/S1PR信号通路的影响[J].中国中医药信息杂志,2022,29(3):85-91.
[6] LI J, HUANG Y, ZHANG Y, et al. S1P/S1PR signaling pathway advancements in autoimmune diseases. Biomol Biomed. 2023;23(6): 922-935.
[7] LOH KC, LEONG WI, CARLSON ME, et al. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS One. 2012;7(5):e37218.
[8] HOU L, ZHANG Z, YANG L, et al. NLRP3 inflammasome priming and activation in cholestatic liver injury via the sphingosine 1-phosphate/S1P receptor 2/Gα((12/13))/MAPK signaling pathway. J Mol Med (Berl). 2021;99(2):273-288.
[9] PIERUCCI F, CHIRCO A, MEACCI E. Irisin is target of sphingosine-1-phosphate/sphingosine-1-phosphate receptor-mediated signaling in skeletal muscle cells. Int J Mol Sci. 2023;24(13):10548.
[10] HODUN K, CHABOWSKI A, BARANOWSKI M. Sphingosine-1-phosphate in acute exercise and training. Scand J Med Sci Sports. 2021;31(5): 945-955.
[11] CORDEIRO AV, SILVA VRR, PAULI JR, et al. The role of sphingosine-1-phosphate in skeletal muscle: physiology, mechanisms, and clinical perspectives. J Cell Physiol. 2019;234(7):10047-10059.
[12] CHEN H, CHEN K, HUANG W, et al. Structure of S1PR2-heterotrimeric G 13 signaling complex. Sci Adv. 2022;8(13):eabn0067.
[13] GREWE JM, KNAPSTEIN PR, DONAT A, et al. The role of sphingosine-1-phosphate in bone remodeling and osteoporosis. Bone Res. 2022; 10(1):34.
[14] EL JAMAL A, BOUGAULT C, MEBAREK S, et al. The role of sphingosine 1-phosphate metabolism in bone and joint pathologies and ectopic calcification. Bone. 2020;130:115087.
[15] CHEN T, GU K, LIN R, et al. The function of sphingosine-1-phosphate receptor 2 (S1PR2) in maintaining intestinal barrier and inducing ulcerative colitis. Bioengineered. 2022;13(5):13703-13717.
[16] WANG N, LI J, ZENG B, et al. Sphingosine-1-phosphate signaling in cardiovascular diseases. Biomolecules. 2023;13(5):818.
[17] TIAN J, MA S, XIE WQ, et al. Sphingosine 1-phosphate and osteoporosis: pathophysiology and therapeutic aspects-a narrative review. Ann Palliat Med. 2021;10(4):4799-4805.
[18] 韦曦华,王泽群,陈靖京,等.鞘氨醇激酶和1-磷酸鞘氨醇及其受体信号在肿瘤微环境中的研究进展[J].药学学报,2023,58(3):571-580.
[19] ISHAY Y, ROTNEMER-GOLINKIN D, ILAN Y. The role of the sphingosine axis in immune regulation: a dichotomy in the anti-inflammatory effects between sphingosine kinase 1 and sphingosine kinase 2-dependent pathways. Int J Immunopathol Pharmacol. 2021;35: 20587384211053274.
[20] GUPTA P, TAIYAB A, HUSSAIN A, et al. Targeting the sphingosine kinase/sphingosine-1-phosphate signaling axis in drug discovery for cancer therapy. Cancers. 2021;13(8):1898.
[21] NG ML, YARLA NS, MENSCHIKOWSKI M, et al. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J Stem Cells. 2018;10(9):119-133.
[22] ZHANG L, DONG Y, WANG Y, et al. Sphingosine-1-phosphate (S1P) receptors: promising drug targets for treating bone-related diseases. J Cell Mol Med. 2020;24(8):4389-4401.
[23] 刘慧,陈慧鸿,廖红兵.破骨细胞衍生的偶联因子鞘氨醇-1-磷酸及血小板衍生生长因子BB对成骨细胞的调节作用[J].中国组织工程研究,2019,23(23):3739-3745.
[24] CRUNKHORN S. Understanding sphingosine-1-phosphate transport. Nat Rev Drug Discov. 2023;22(7):538.
[25] CZUBOWICZ K, JĘŚKO H, WENCEL P, et al. The role of ceramide and sphingosine-1-phosphate in alzheimer’s disease and other neurodegenerative disorders. Mol Neurobiol. 2019;56(8):5436-5455.
[26] CHEN H, WANG J, ZHANG C, et al. Sphingosine 1-phosphate receptor, a new therapeutic direction in different diseases. Biomed Pharmacother. 2022;153:113341.
[27] XU X, HAN Y, ZHU T, et al. The role of SphK/S1P/S1PR signaling pathway in bone metabolism. Biomed Pharmacother. 2023;169:115838.
[28] ZANIN M, GERMINARIO E, DALLA LIBERA L, et al. Trophic action of sphingosine 1-phosphate in denervated rat soleus muscle. Am J Physiol Cell Physiol. 2008;294(1):C36-C46.
[29] BONDì M, GERMINARIO E, PIRAZZINI M, et al. Ablation of S1P3 receptor protects mouse soleus from age-related drop in muscle mass, force, and regenerative capacity. Am J Physiol Cell Physiol. 2017; 313(1):C54-C67.
[30] 任翔宇,沈飞,金玲,等.运动促进骨骼肌健康的新视角:基于Rac1/PAK1/p38 MAPK信号通路改善肌生成和糖代谢的研究进展与展望[J].中国体育科技,2023,59(5):79-87.
[31] 陈昱圻,郭昌龙,袁国红,等.衰老个体骨骼肌卫星细胞的研究进展[J].中国老年学杂志,2022,42(17):4354-4360.
[32] TAN-CHEN S, GUITTON J, BOURRON O, et al. Sphingolipid metabolism and signaling in skeletal muscle: from physiology to physiopathology. Front Endocrinol (Lausanne). 2020;11:491.
[33] BERNACCHIONI C, GHINI V, SQUECCO R, et al. Role of sphingosine 1-phosphate signalling axis in muscle atrophy induced by TNFα in C2C12 myotubes. Int J Mol Sci. 2021;22(3):1280.
[34] BERNACCHIONI C, SQUECCO R, GAMBERI T, et al. S1P signalling axis is necessary for adiponectin-directed regulation of electrophysiological properties and oxidative metabolism in C2C12 myotubes. Cells. 2022; 11(4):713.
[35] GERMINARIO E, BONDÌ M, BLAAUW B, et al. Reduction of circulating sphingosine-1-phosphate worsens mdx soleus muscle dystrophic phenotype. Exp Physiol. 2020;105(11):1895-1906.
[36] MEACCI E, PIERUCCI F, GARCIA-GIL M. Skeletal muscle and COVID-19: the potential involvement of bioactive sphingolipids. Biomedicines. 2022;10(5):1068.
[37] GERMINARIO E, PERON S, TONIOLO L, et al. S1P2 receptor promotes mouse skeletal muscle regeneration. J Appl Physiol (1985). 2012; 113(5):707-713.
[38] SONG ZW , JIN CL , YE M , GAO CQ , et al. Lysine inhibits apoptosis in satellite cells to govern skeletal muscle growth via the JAK2-STAT3 pathway. Food Funct. 2020;11(5):3941-3951.
[39] DE LA GARZA-RODEA AS, MOORE SA, ZAMORA-PINEDA J, et al. Sphingosine phosphate lyase is upregulated in duchenne muscular dystrophy, and its inhibition early in life attenuates inflammation and dystrophy in mdx mice. Int J Mol Sci. 2022;23(14):7579.
[40] CHEN H, AHMED S, ZHAO H, et al. Structural and functional insights into Spns2-mediated transport of sphingosine-1-phosphate. Cell. 2023;186(12):2644-2655.e16.
[41] CENCETTI F, BRUNO G, BERNACCHIONI C, et al. Sphingosine 1-phosphate lyase blockade elicits myogenic differentiation of murine myoblasts acting via Spns2/S1P2 receptor axis. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(9):158759.
[42] BRUNO G, CENCETTI F, BERNACCHIONI C, et al. Bradykinin mediates myogenic differentiation in murine myoblasts through the involvement of SK1/Spns2/S1P2 axis. Cell Signal. 2018;45:110-121.
[43] 徐帅,徐道明,沈飞.肌骨系统中运动干预肌肉与骨骼交互功能的机制研究进展[J].山东体育学院学报,2022,38(2):91-99.
[44] BERNACCHIONI C, CENCETTI F, BLESCIA S, et al. Sphingosine kinase/sphingosine 1-phosphate axis: a new player for insulin-like growth factor-1-induced myoblast differentiation. Skelet Muscle. 2012;2(1):15.
[45] DONATI C, MEACCI E, NUTI F, et al. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 2005;19(3):449-451.
[46] BERGMAN BC, BROZINICK JT, STRAUSS A, et al. Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia. 2016;59(4):785-798.
[47] BANITALEBI E, GHARAKHANLOU R, GHATREHSAMANI K, et al. The effect of resistance training on plasma S1P level and gene expression of S1P1, 2, 3 receptors in male Wistar rats. Minerva Endocrinol. 2013;38(4): 395-400.
[48] 王倩,傅力.鞘氨醇激酶——运动改善胰岛素抵抗的新靶点[J].中国运动医学杂志,2016,35(12):1162-1164.
[49] BŁACHNIO-ZABIELSKA A, BARANOWSKI M, ZABIELSKI P, et al. Effect of exercise duration on the key pathways of ceramide metabolism in rat skeletal muscles. J Cell Biochem. 2008;105(3):776-784.
[50] BARANOWSKI M, CHARMAS M, DŁUGOŁĘCKA B, et al. Exercise increases plasma levels of sphingoid base-1 phosphates in humans. Acta Physiol (Oxf). 2011;203(3):373-380.
[51] BARANOWSKI M, BŁACHNIO-ZABIELSKA AU, Charmas M, et al. Exercise increases sphingoid base-1-phosphate levels in human blood and skeletal muscle in a time- and intensity-dependent manner. Eur J Appl Physiol. 2015;115(5):993-1003.
[52] BERGMAN BC, BROZINICK JT, STRAUSS A, et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab. 2015;309(4):E398-E408.
[53] BARANOWSKI M, GÓRSKI J, KLAPCINSKA B, et al. Ultramarathon run markedly reduces plasma sphingosine-1-phosphate concentration. Int J Sport Nutr Exerc Metab. 2014;24(2):148-156.
[54] BŁACHNIO-ZABIELSKA A, ZABIELSKI P, BARANOWSKI M, et al. Aerobic training in rats increases skeletal muscle sphingomyelinase and serine palmitoyltransferase activity, while decreasing ceramidase activity. Lipids. 2011;46(3):229-238.
[55] KSIĄŻEK M, CHARMAS M, KLUSIEWICZ A, et al. Endurance training selectively increases high-density lipoprotein-bound sphingosine-1-phosphate in the plasma. Scand J Med Sci Sports. 2018;28(1):57-64.
[56] LIU M, FREJ C, LANGEFELD CD, et al. Plasma apoM and S1P levels are inversely associated with mortality in African Americans with type 2 diabetes mellitus. J Lipid Res. 2019;60(8):1425-1431.
[57] JØRGENSEN MLK, KjØLHEDE T, DALGAS U, et al. Plasma brain-derived neurotrophic factor (BDNF) and sphingosine-1-phosphat (S1P) are NOT the main mediators of neuroprotection induced by resistance training in persons with multiple sclerosis-A randomized controlled trial. Mult Scler Relat Disord. 2019;31:106-111.
[58] YAFASOVA A, MANDRUP CM, EGELUND J, et al. Effect of menopause and exercise training on plasma apolipoprotein M and sphingosine-1-phosphate. J Appl Physiol (1985). 2019;126(1):214-220.
[59] LIU X, HOENE M, YIN P, et al. Quality control of serum and plasma by quantification of (4E,14Z)-sphingadienine-C18-1-phosphate uncovers common preanalytical errors during handling of whole blood. Clin Chem. 2018;64(5):810-819.
[60] DUBÉ JJ, AMATI F, TOLEDO FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147-1156.
[61] BARANOWSKI M, ZABIELSKI P, BLACHNIO A, et al. Effect of exercise duration on ceramide metabolism in the rat heart. Acta Physiol (Oxf). 2008;192(4):519-529.
[62] SILVA VR, KATASHIMA CK, BUENO SILVA CG, et al. Hypothalamic S1P/S1PR1 axis controls energy homeostasis in middle-aged rodents: the reversal effects of physical exercise [published correction appears in Aging (Albany NY). 2020 May 30;12(10):10000]. Aging (Albany NY). 2016;9(1):142-155.
[63] LEE J, SAVAGE H, MAEGAWA S, et al. Exercise promotes pro-apoptotic ceramide signaling in a mouse melanoma model. Cancers (Basel). 2022;14(17):4306.
[64] HUO F, LIU Q, LIU H. Contribution of muscle satellite cells to sarcopenia. Front Physiol. 2022;13:892749.
[65] SABA JD, DE LA GARZA-RODEA AS. S1P lyase in skeletal muscle regeneration and satellite cell activation: exposing the hidden lyase. Biochim Biophys Acta. 2013;1831(1):167-175.
[66] 王兰兰,薛惠天,孙梦龙,等.推拿?法对兔骨骼肌急性钝挫伤组织TNF-α及SphK1、S1PR3表达的影响[J].中国中医药信息杂志, 2023,30(6):129-134.
[67] PIERUCCI F, FRATI A, BATTISTINI C, et al. Involvement of released sphingosine 1-phosphate/sphingosine 1-phosphate receptor axis in skeletal muscle atrophy. Biochim Biophys Acta Mol Basis Dis. 2018; 1864(12):3598-3614.
[68] 贾单单, 田振军, DU S. 8周间歇运动激活LIF-LIFR-STAT3信号和诱导骨骼肌细胞增殖[J].北京体育大学学报,2017,40(10):44-49.
[69] TRENERRY MK, DELLA GATTA PA, LARSEN AE, et al. Impact of resistance exercise training on interleukin-6 and JAK/STAT in young men. Muscle Nerve. 2011;43(3):385-392.
|