[1] LIN L, ZHANG MX, ZHANG L, et al. Autophagy, Pyroptosis, and Ferroptosis: New Regulatory Mechanisms for Atherosclerosis. Front Cell Dev Biol. 2021;9:809955.
[2] FAN J, WATANABE T. Atherosclerosis: Known and unknown. Pathol Int. 2022;72(3):151-160.
[3] WU G, YU G, ZHENG M, et al. Recent Advances for Dynamic-Based Therapy of Atherosclerosis. Int J Nanomedicine. 2023;18:3851-3878.
[4] XIN S, SCHICK JA. PUFAs dictate the balance of power in ferroptosis. Cell Calcium. 2023;110:102703.
[5] BAI T, LI M, LIU Y, et al. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92-102.
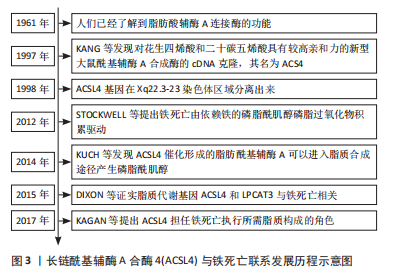
[6] HATCH MD, STUMPF PK. Fat metabolism in higher plants. XVI. Acetyl coenzyme A carboxylase and acyl coenzyme A-malonyl coenzyme A transcarboxylase from wheat germ. J Biol Chem. 1961;236:2879-2885.
[7] KANG MJ, FUJINO T, SASANO H, et al. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc Natl Acad Sci U S A. 1997;94(7):2880-2884.
[8] PICCINI M, VITELLI F, BRUTTINI M, et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics. 1998; 47(3):350-358.
[9] MASHEK DG, LI LO, COLEMAN RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2(4):465-476.
[10] KÜCH EM, VELLARAMKALAYIL R, ZHANG I, et al. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim Biophys Acta. 2014;1841(2):227-239.
[11] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5): 1060-1072.
[12] DIXON SJ, WINTER GE, MUSAVI LS, et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10(7):1604-1609.
[13] YUAN H, LI X, ZHANG X, et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016; 478(3):1338-1343.
[14] KAGAN VE, MAO G, QU F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017; 13(1): 81-90.
[15] CHEN J, DING C, CHEN Y, et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Lett. 2021;502:154-165.
[16] KUWATA H, HARA S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019;144:106363.
[17] WU Z, SUN J, LIAO Z, et al. An update on the therapeutic implications of long-chain acyl-coenzyme A synthetases in nervous system diseases. Front Neurosci. 2022;16:1030512.
[18] HOU J, JIANG C, WEN X, et al. ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics. Front Pharmacol. 2022;13:949863.
[19] RADIF Y, NDIAYE H, KALANTZI V, et al. The endogenous subcellular localisations of the long chain fatty acid-activating enzymes ACSL3 and ACSL4 in sarcoma and breast cancer cells. Mol Cell Biochem. 2018; 448(1-2):275-286.
[20] KILLION EA, REEVES AR, EL AZZOUNY MA, et al. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol Metab. 2018;9:43-56.
[21] SINGH AB, KAN CFK, KRAEMER FB, et al. Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2019;316(5):E880-E894.
[22] SUN W, WU X, YU P, et al. LncAABR07025387.1 Enhances Myocardial Ischemia/Reperfusion Injury Via miR-205/ACSL4-Mediated Ferroptosis. Front Cell Dev Biol. 2022;10:672391.
[23] SHI L, SONG Z, LI Y, et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am J Transplant. 2023;23(1):11-25.
[24] LU Y, CHAN YT, TAN HY, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41(1):3.
[25] MA LL, LIANG L, ZHOU D, et al. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68(1):165-173.
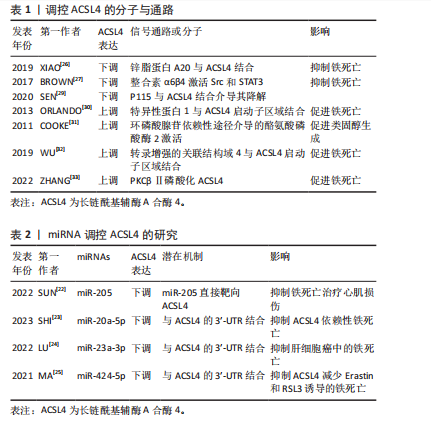
[26] XIAO FJ, ZHANG D, WU Y, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun. 2019;515(3):448-454.
[27] BROWN CW, AMANTE JJ, GOEL HL, et al. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol. 2017;216(12):4287-4297.
[28] LI YJ, FAHRMANN JF, AFTABIZADEH M, et al. Fatty acid oxidation protects cancer cells from apoptosis by increasing mitochondrial membrane lipids. Cell Rep. 2022;39(9):110870.
[29] SEN P, KAN CFK, SINGH AB, et al. Identification of p115 as a novel ACSL4 interacting protein and its role in regulating ACSL4 degradation. J Proteomics. 2020;229:103926.
[30] ORLANDO U, COOKE M, CORNEJO MACIEL F, et al. Characterization of the mouse promoter region of the acyl-CoA synthetase 4 gene: role of Sp1 and CREB. Mol Cell Endocrinol. 2013;369(1-2):15-26.
[31] COOKE M, ORLANDO U, MALOBERTI P, et al. Tyrosine phosphatase SHP2 regulates the expression of acyl-CoA synthetase ACSL4. J Lipid Res. 2011;52(11):1936-1948.
[32] WU J, MINIKES AM, GAO M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769): 402-406.
[33] ZHANG HL, HU BX, LI ZL, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24(1):88-98.
[34] CHEN X, LI J, KANG R, et al. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054-2081.
[35] STOCKWELL BR, FRIEDMANN ANGELI JP, BAYIR H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273-285.
[36] LIU J, KANG R, TANG D. Signaling pathways and defense mechanisms of ferroptosis. Febs J. 2022;289(22):7038-7050.
[37] DOLL S, PRONETH B, TYURINA YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017; 13(1):91-98.
[38] TOMITSUKA Y, IMAEDA H, ITO H, et al. Gene deletion of long-chain acyl-CoA synthetase 4 attenuates xenobiotic chemical-induced lung injury via the suppression of lipid peroxidation. Redox Biol. 2023;66:102850.
[39] WANG Y, YU R, WU L, et al. Hydrogen sulfide guards myoblasts from ferroptosis by inhibiting ALOX12 acetylation. Cell Signal. 2021; 78:109870.
[40] LI FJ, LONG HZ, ZHOU ZW, et al. System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol. 2022;13:910292.
[41] WEI Y, LV H, SHAIKH AB, et al. Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim Biophys Acta Gen Subj. 2020;1864(4): 129539.
[42] CHU B, KON N, CHEN D, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21(5):579-591.
[43] INGOLD I, BERNDT C, SCHMITT S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018; 172(3):409-422.e421.
[44] MAGTANONG L, MUELLER GD, WILLIAMS KJ, et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem Biol. 2022;29(9): 1409-1418.e1406.
[45] MUSSBACHER M, SCHOSSLEITNER K, KRAL-POINTNER JB, et al. More than Just a Monolayer: the Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Curr Atheroscler Rep. 2022;24(6): 483-492.
[46] VINCHI F, PORTO G, SIMMELBAUER A, et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2020;41(28):2681-2695.
[47] ZHANG M, YU Z, ZHAO L, et al. Long non-coding RNA PVT1 regulates atherosclerosis progression via the microRNA-106b-5p/ACSL4 axis. Biochem Biophys Res Commun. 2023;667:170-179.
[48] ZHOU Y, ZHOU H, HUA L, et al. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic Biol Med. 2021;171:55-68.
[49] LIU X, WU J, TIAN R, et al. Targeting foam cell formation and macrophage polarization in atherosclerosis: The Therapeutic potential of rhubarb. Biomed Pharmacother. 2020;129:110433.
[50] YANG Z, SHI J, CHEN L, et al. Role of Pyroptosis and Ferroptosis in the Progression of Atherosclerotic Plaques. Front Cell Dev Biol. 2022;10:811196.
[51] YE Y, CHEN A, LI L, et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022; 102(6):1259-1275.
[52] SU EJ, LAWRENCE DA. Diabetes and the treatment of ischemic stroke. J Diabetes Complications. 2022;36(11):108318.
[53] XU Y, LI K, ZHAO Y, et al. Role of Ferroptosis in Stroke. Cell Mol Neurobiol. 2023;43(1):205-222.
[54] WANG P, CUI Y, REN Q, et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021;12(5):447.
[55] CUI Y, ZHANG Y, ZHAO X, et al. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312-321.
[56] TUO Q Z, LIU Y, XIANG Z, et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther. 2022;7(1):59.
[57] LIU H, ZHAO Z, YAN M, et al. Calycosin decreases cerebral ischemia/reperfusion injury by suppressing ACSL4-dependent ferroptosis. Arch Biochem Biophys. 2023;734:109488.
[58] LYU N, LI X. Sevoflurane Postconditioning Attenuates Cerebral Ischemia-Reperfusion Injury by Inhibiting SP1/ACSL4-Mediated Ferroptosis. Hum Exp Toxicol. 2023;42:9603271231160477.
[59] XIE Y, WANG Y, ZHAO L, et al. Identification of potential biomarkers and immune cell infiltration in acute myocardial infarction (AMI) using bioinformatics strategy. Bioengineered. 2021;12(1):2890-2905.
[60] KONIJNENBERG LSF, DAMMAN P, DUNCKER DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116(4):787-805.
[61] ZHAO WK, ZHOU Y, XU TT, et al. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021;2021:9929687.
[62] BULLUCK H, ROSMINI S, ABDEL-GADIR A, et al. Residual Myocardial Iron Following Intramyocardial Hemorrhage During the Convalescent Phase of Reperfused ST-Segment-Elevation Myocardial Infarction and Adverse Left Ventricular Remodeling. Circ Cardiovasc Imaging. 2016;9(10): e004940.
[63] HU Y, LI Q, WANG Y. Serum ACSL4 levels in patients with ST-segment elevation myocardial infarction (STEMI) and its association with one-year major adverse cardiovascular events (MACE): A prospective cohort study. Medicine (Baltimore). 2024;103(2):e36870.
[64] YU Q, ZHANG N, GAN X, et al. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomedicine. 2023;119:154999.
[65] TANG LJ, LUO XJ, TU H, et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(2): 401-410.
[66] ZHANG F, LI Z, GAO P, et al. HJ11 decoction restrains development of myocardial ischemia-reperfusion injury in rats by suppressing ACSL4-mediated ferroptosis. Front Pharmacol. 2022;13:1024292.
[67] QIU M, YAN W, LIU M. YAP Facilitates NEDD4L-Mediated Ubiquitination and Degradation of ACSL4 to Alleviate Ferroptosis in Myocardial Ischemia-Reperfusion Injury. Can J Cardiol. 2023;39(11):1712-1727.
[68] ZHANG Y, XIN L, XIANG M, et al. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother. 2022;145:112423.
[69] SHA R, XU Y, YUAN C, et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine. 2021;71:103560.
[70] KUNG YA, CHIANG HJ, LI ML, et al. Acyl-Coenzyme A Synthetase Long-Chain Family Member 4 Is Involved in Viral Replication Organelle Formation and Facilitates Virus Replication via Ferroptosis. mBio. 2022; 13(1):e0271721.
[71] ASKARI B, KANTER JE, SHERRID AM, et al. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes. 2007;56(4):1143-1152.
[72] CHEN J, YANG L, GENG L, et al. Inhibition of Acyl-CoA Synthetase Long-Chain Family Member 4 Facilitates Neurological Recovery After Stroke by Regulation Ferroptosis. Front Cell Neurosci. 2021;15:632354.
[73] FAN Z, CAI L, WANG S, et al. Baicalin Prevents Myocardial Ischemia/Reperfusion Injury Through Inhibiting ACSL4 Mediated Ferroptosis. Front Pharmacol. 2021;12:628988.
[74] DUAN J, WANG Z, DUAN R, et al. Therapeutic targeting of hepatic ACSL4 ameliorates NASH in mice. Hepatology. 2022;75(1):140-153.
[75] CASTILLO AF, ORLANDO UD, MALOBERTI PM, et al. New inhibitor targeting Acyl-CoA synthetase 4 reduces breast and prostate tumor growth, therapeutic resistance and steroidogenesis. Cell Mol Life Sci, 2021;78(6):2893-2910.
[76] XU Y, LI X, CHENG Y, et al. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. Faseb J. 2020;34(12): 16262-16275. |