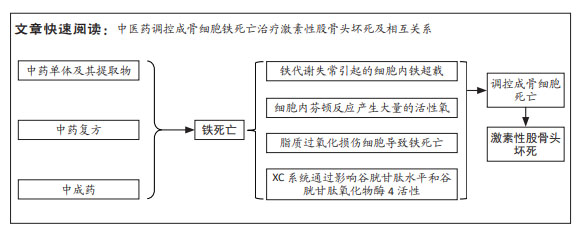
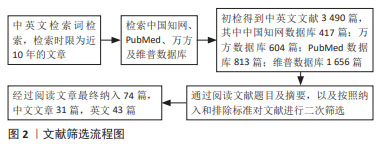
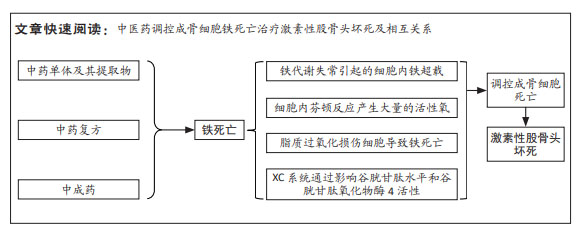
[1] ZHANG S, WANG C, SHI L, et al. Beware of steroid-induced avascular necrosis of the femoral head in the treatment of COVID-19-experience and lessons from the SARS epidemic. Drug Des Devel Ther. 2021;15:983-995.
[2] LIU Q, WU Y, LI S, et al. Ursolic acid alleviates steroid-induced avascular necrosis of the femoral head in mouse by inhibiting apoptosis and rescuing osteogenic differentiation. Toxicol Appl Pharmacol. 2023;475:116649.
[3] XI H, TAO W, JIAN Z, et al. Levodopa attenuates cellular apoptosis in steroid-associated necrosis of the femoral head. Exp Ther Med. 2017;13(1):69-74.
[4] 王小龙,韩超前,赵晓娜,等.普伐他汀能否降低早期兔激素诱导性股骨头坏死发生的风险[J].中国组织工程研究,2018,22(32):5097-5103.
[5] 尚征亚,曹林忠,张翼,等.中药单体调控成骨相关信号通路治疗激素性股骨头坏死的研究进展[J].中国实验方剂学杂志,2023,29(18):229-240.
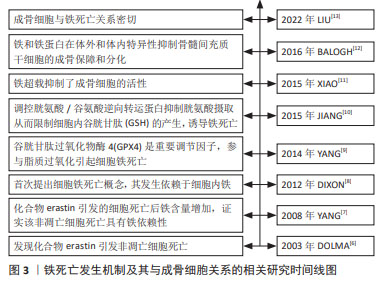
[6] DOLMA S, LESSNICK SL, HAHN WC, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285-296.
[7] YANG WS, STOCKWELL BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234-245.
[8] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072.
[9] YANG WS, SRIRAMARATNAM R, WELSCH M E, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317-331.
[10] JIANG L, KON N, LI T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57-62.
[11] XIAO W, BEIBEI F, GUANGSI S, et al. Iron overload increases osteoclastogenesis and aggravates the effects of ovariectomy on bone mass. J Endocrinol. 2015;226(3):121-134.
[12] BALOGH E, TOLNAI E, NAGY BJ, et al. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta. 2016;1862(9):1640-1649.
[13] LIU P, WANG W, LI Z, et al. Ferroptosis: a new regulatory mechanism in osteoporosis. Oxid Med Cell Longev. 2022;2022:2634431.
[14] CUI D, ZHAO D, HUANG S. Beneficial contribution of a safflower (Carthamus tinctorius L.) polysaccharide on steroid-induced avascular necrosis of the femoral head in rats. Int J Biol Macromol. 2019;123:581-586.
[15] WANG B, GONG S, SHAO W, et al. Comprehensive analysis of pivotal biomarkers, immune cell infiltration and therapeutic drugs for steroid-induced osteonecrosis of the femoral head. Bioengineered. 2021;12(1):5971-5984.
[16] 孙懿,赵海燕,成杰,等.激素性股骨头坏死发生机制的研究进展[J].中国矫形外科杂志,2023,31(1):58-62.
[17] KRATOCHVILOVA A, RAMESOVA A, VESELA B, et al. Impact of fasl stimulation on sclerostin expression and osteogenic profile in IDG-SW3 osteocytes. Biology (Basel). 2021;10(8):757.
[18] 丁香莹,梁敏.凋亡在糖皮质激素性骨质疏松症中的研究进展[J].中国骨质疏松杂志,2017,23(12):1660-1663.
[19] MUTIJIMA E, DE MAERTELAER V, DEPREZ M, et al. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: its specificity and its distribution. Clin Rheumatol. 2014;33(12):1791-1795.
[20] 万超超,曹林忠,王多贤,等.成骨细胞铁代谢异常的研究进展[J].中国骨质疏松杂志,2023,29(5):691-694.
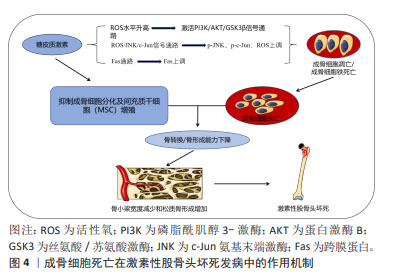
[21] PENG P, NIE Z, SUN F, et al. Glucocorticoids induce femoral head necrosis in rats through the ROS/JNK/c-Jun pathway. FEBS Open Bio. 2021;11(1):312-321.
[22] XIE B, ZENG Z, LIAO S, et al. Kaempferol ameliorates the inhibitory activity of dexamethasone in the osteogenesis of MC3T3-E1 cells by JNK and p38-MAPK pathways. Front Pharmacol. 2021;12:739326.
[23] XING Q, FENG J, ZHANG X. Glucocorticoids suppressed osteoblast differentiation by decreasing Sema3A expression via the PIK3/Akt pathway.Exp Cell Res. 2021;403(1):112595.
[24] LI J, CAO F, YIN H L, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88.
[25] YANG WS, KIM KJ, GASCHLER MM, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113(34):E4966-E4975.
[26] LUO C, XU W, TANG X, et al. Canonical Wnt signaling works downstream of iron overload to prevent ferroptosis from damaging osteoblast differentiation. Free Radic Biol Med. 2022;188:337-350.
[27] HU W, ZHOU C, JING Q, et al. FTH promotes the proliferation and renders the HCC cells specifically resist to ferroptosis by maintaining iron homeostasis. Cancer Cell Int. 2021;21(1):709.
[28] 谢恒,顾叶,顾赢楚,等.骨骼疾病中的铁死亡:骨质疏松治疗靶点[J].中国组织工程研究,2024,28(16):2613-2618.
[29] SHANG Y, LUO M, YAO F, et al. Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal. 2020;72:109633.
[30] FANG Y, CHEN X, TAN Q, et al. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Cent Sci. 2021;7(6):980-989.
[31] SU L J, ZHANG J H, GOMEZ H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843.
[32] ABE C, MIYAZAWA T. Current use of fenton reaction in drugs and food. Molecules. 2022;27(17):5451.
[33] LIU J, KANG R, TANG D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289(22):7038-7050.
[34] TAO H, GEe G, LIANG X, et al. ROS signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin (Shanghai). 2020; 52(10):1055-1062.
[35] BAO J, YAN Y, ZUO D, et al. Iron metabolism and ferroptosis in diabetic bone loss: from mechanism to therapy. Front Nutr. 2023;10:1178573.
[36] YANG WS, KIM KJ, GASCHLER MM, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016; 113(34):E4966-E4975.
[37] YAN C, ZHANG J, AN F, et al. Research progress of ferroptosis regulatory network and bone remodeling in osteoporosis. Front Public Health. 2022;10:910675.
[38] XIONG Y, CHEN L, LIN Z, et al. The regulatory role of ferroptosis in bone homeostasis. Stem Cells Int. 2022;2022:3568597.
[39] MA J, WANG A, ZHANG H, et al. Iron overload induced osteocytes apoptosis and led to bone loss in Hepcidin (-/-) mice through increasing sclerostin and RANKL/OPG. Bone. 2022;164:116511.
[40] JIANG Z, WANG H, QI G, et al. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: an in vitro and in vivo study. IUBMB Life. 2022;74(11):1052-1069.
[41] LIU J, JIANG G, HE P, et al. Mechanism of ferroptosis in traditional chinese medicine for clinical treatment: a review. Front Pharmacol. 2022;13:1108836.
[42] 陈卫衡,何伟,童培建,等.股骨头坏死中医辨证标准(2019年版)[J].中医正骨,2019,31(6):1-2.
[43] XIE D, XU Y, CAIW, et al. Icariin promotes osteogenic differentiation by upregulating alpha-enolase expression. Biochem Biophys Rep. 2023;34:101471.
[44] 李嘉骏,夏天,刘佳敏,等.淫羊藿苷调控成骨信号相关通路治疗激素性股骨头缺血性坏死的分子机制[J].中国组织工程研究,2022,26(5):780-785.
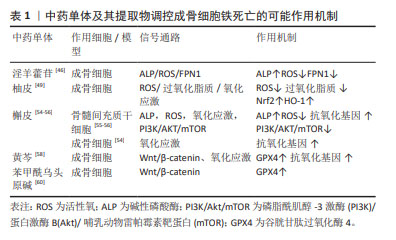
[45] 付殷,刘宇洲,胡晓阳,等.基于铁死亡通路研究淫羊藿苷对大鼠成骨细胞增殖分化的影响[J].时珍国医国药,2022,33(9):2100-2103.
[46] REN GW, WEN SB, HAN J, et al. Network-based pharmacology and bioinformatics study on the mechanism of action of gujiansan in the treatment of steroid-induced avascular necrosis of the femoral head. Biomed Res Int. 2022;2022:8080679.
[47] 李泽林,孙海飚.柚皮素治疗骨质疏松的作用机制研究[J].中国骨质疏松杂志,2023,29(6):932-936.
[48] PAN Z, HE Q, ZENG J, et al. Naringenin protects against iron overload-induced osteoarthritis by suppressing oxidative stress. Phytomedicine. 2022;105:154330.
[49] XU S, WU B, ZHONG B, et al. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered. 2021;12(2):10924-10934.
[50] 肖振,李盛华.激素性股骨头坏死血管新生相关基因的鉴定及其靶向中药成分筛选验证[J].中草药,2023,54(5):1526-1539.
[51] 刘洪柱,李垚,陈鑫,等.沙棘总黄酮、槲皮素对鸡成骨细胞碱性磷酸酶活性作用机制的体外研究[J].动物营养学报,2011,23(8):1378-1385.
[52] MESSER JG, HOPKINS RG, KIPP DE. Quercetin metabolites up-regulate the antioxidant response in osteoblasts isolated from fetal rat calvaria. J Cell Biochem. 2015;116(9):1857-1866.
[53] LI X, ZENG J, LIU Y, et al. Inhibitory effect and mechanism of action of quercetin and quercetin diels-alder anti-dimer on erastin-induced ferroptosis in bone marrow-derived mesenchymal stem cells. Antioxidants (Basel). 2020;9(3):205.
[54] LAN D, QI S, YAO C, et al. Quercetin protects rat BMSCs from oxidative stress via ferroptosis. J Mol Endocrinol. 2022;69(3):401-413.
[55] 王雅芳,李婷,唐正海,等.中药黄芩的化学成分及药理研究进展[J].中华中医药学刊,2015,33(1):206-211.
[56] 庄文德,郭浩华,陈振,等.铁死亡调控在骨质疏松症中的作用机制及中药干预研究[J].中国骨质疏松杂志,2023,29(6):890-896.
[57] 段丽颖,王绍宁,那铎,等.双黄岑素氧钒配合物的抗氧化性和抑菌活性[J].沈阳药科大学学报,2018,35(7):581-586.
[58] 徐晶.苯甲酰乌头原碱对Erastin诱导成骨细胞铁死亡的影响及潜在机制[D].南京:南京中医药大学,2022.
[59] 帅波,杨功旭,沈霖,等.加味青娥丸对激素性股骨头坏死小鼠局部1,25(OH)2D3/VDR mRNA/RAS信号转导通路的影响[J].中国中医骨伤科杂志,2017,25(7):1-5.
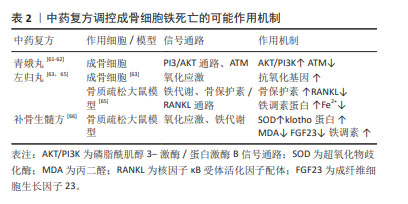
[60] 翁泽斌,颜翠萍,张志杰,等.不同炮制品入药的青娥丸含药血清对人成骨细胞增殖、分化及矿化的影响[J].中国实验方剂学杂志,2015, 21(6):165-168.
[61] 邸学士,陈昭,贾育松,等.基于网络药理学探讨青娥丸治疗绝经后骨质疏松症的作用机制[J].中国骨质疏松杂志,2021,27(3):364-371.
[62] HAO J, BEI J, LI Z, et al. Qing’e pill inhibits osteoblast ferroptosis via ATM serine/threonine kinase (ATM) and the PI3K/AKT pathway in primary osteoporosis. Front Pharmacol. 2022;13:902102.
[63] 乔久涛,关德宏,王冬艳,等.左归丸对成骨细胞氧化应激损伤的保护作用[J].中国组织工程研究,2020,24(7):1052-1056.
[64] 胡阳,宋敏,李凯等.左归丸及其组方治疗绝经后骨质疏松症的基础研究进展[J].中华中医药学刊,41(12):86-89.
[65] 刘梅洁,吴佳莹,李艳,等.左归丸对骨质疏松症模型大鼠铁过载的影响[J].中医杂志,2018,59(9):777-780.
[66] 章轶立,方圣杰,李秋月,等.补骨生髓方对骨质疏松症模型大鼠氧化应激及铁死亡相关指标的影响[J].中国中医药信息杂志,2022,29(4):75-79.
[67] 程韶,舒冰,赵永见,等.氧化应激对骨重建的影响[J].中国骨质疏松杂志,2019,25(10):1478-1482.
[68] 乔明珠,吕浩,胡芷苜,等.基于Wnt/β-catenin信号通路的复方补肾活血颗粒对骨髓间充质干细胞成骨、成脂分化的影响[J].中国中医药信息杂志,2023,30(11):107-113.
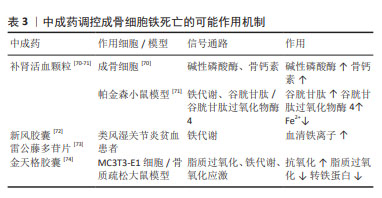
[69] 王子文,王久香,吕浩,等.复方补肾活血颗粒含药血清通过Trb3调控hBMSCs成骨成脂分化[J].中国骨质疏松杂志,2023,29(4):544-549.
[70] 刘慧,许兵,方剑利,等.补肾活血颗粒对去势大鼠血生化的影响[J].中华中医药杂志,2012,27(5):1435-1438.
[71] 李晨,王鹏,王亮,等.补肾活血颗粒对亚急性帕金森病模型小鼠脑黑质多巴胺神经元铁死亡的影响[J].中医杂志,2022,63(15):1463-1469.
[72] 徐慧敏.基于OPG/RANKL/RANK系统探讨新风胶囊对RA患者骨代谢影响及其作用机制[D].合肥:安徽中医药大学,2018.
[73] 孙艳秋,刘健,黄旦,等.不同雷公藤制剂对类风湿关节炎贫血患者的疗效及其机制[J].中国免疫学杂志,2020,36(3):360-364.
[74] 李超,赵剑波,陈俊雅,等.金天格胶囊对H2O2诱导的小鼠成骨细胞MC3T3-E1氧化应激损伤及炎症因子的作用[J].中国骨质疏松杂志, 2022,28(10):1448-1452. |