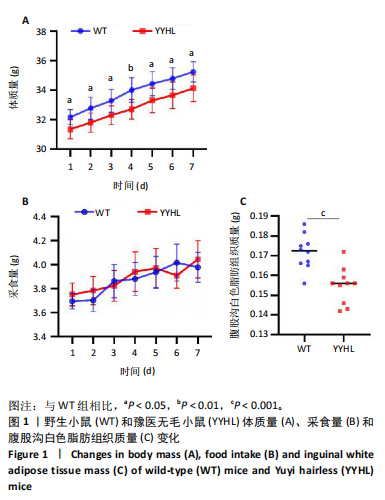
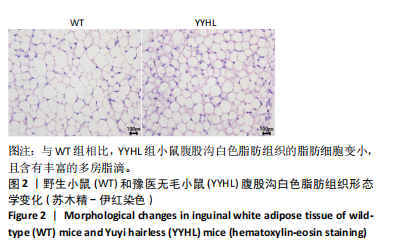
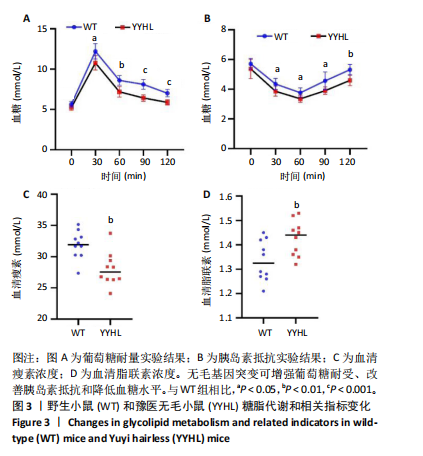
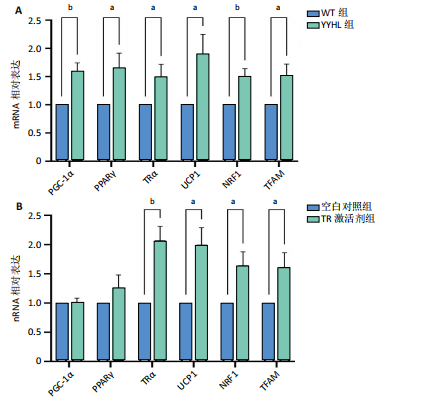
[1] APOVIAN CM, RIFFENBURG KM. Perspectives on the global obesity epidemic. Curr Opin Endocrinol Diabetes Obes. 2017;24(5):307-309.
[2] PICHÉ ME, TCHERNOF A, DESPRÉS JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126(11):1477-1500.
[3] CHANDRASEKARAN P, WEISKIRCHEN R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int J Mol Sci. 2024;25(3):1882.
[4] GANGULI M, BEER JC, ZMUDA JM, et al. Aging, Diabetes, Obesity, and Cognitive Decline: A Population-Based Study. J Am Geriatr Soc. 2020;68(5):991-998.
[5] TIAN X, CHEN S, WANG P, et al. Insulin resistance mediates obesity-related risk of cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):289.
[6] GESTA S, TSENG YH, KAHN CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242-256.
[7] JOHNSON JM, PETERLIN AD, BALDERAS E, et al. Mitochondrial phosphatidylethanolamine modulates UCP1 to promote brown adipose thermogenesis. Sci Adv. 2023;9(8):eade7864.
[8] BARTELT A, HEEREN J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24-36.
[9] WALCZAK K, SIEMINSKA L. Obesity and Thyroid Axis. Int J Environ Res Public Health. 2021;18(18):9434.
[10] MA Y, SHEN S, YAN Y, et al. Adipocyte Thyroid Hormone β Receptor-Mediated Hormone Action Fine-tunes Intracellular Glucose and Lipid Metabolism and Systemic Homeostasis. Diabetes. 2023;72(5):562-574.
[11] BRENT GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035-3043.
[12] LIU YY, SCHULTZ JJ, BRENT GA. A thyroid hormone receptor alpha gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003; 278(40):38913-38920.
[13] AFFORTIT C, BLANC F, NASR J, et al. A disease-associated mutation in thyroid hormone receptor α1 causes hearing loss and sensory hair cell patterning defects in mice. Sci Signal. 2022;15(738):eabj4583.
[14] KUSHCHAYEVA YS, STARTZELL M, COCHRAN E, et al. Thyroid Hormone Effects on Glucose Disposal in Patients With Insulin Receptor Mutations. J Clin Endocrinol Metab. 2020;105(3):e158-e171.
[15] SEGOVIA JC, LIN YC, AALA W, et al. Atrichia with papular lesions in a 1-year-old girl resulting from a new homozygous nonsense pathogenic variant in the hairless gene. Clin Exp Dermatol. 2023;48(5):536-538.
[16] BENAVIDES F, OBERYSZYN TM, VANBUSKIRK AM, et al. The hairless mouse in skin research. J Dermatol Sci. 2009;53(1):10-18.
[17] GAO J, LI Y, GUAN Y, et al. The accelerated aging skin in rhino-like SHJHhr mice. Exp Dermatol. 2022;31(10):1597-1606.
[18] BAUR R, SHANE HL, WEATHERLY LM, et al. Exposure to the immunomodulatory chemical triclosan differentially impacts immune cell populations in the skin of haired (BALB/c) and hairless (SKH1) mice. Toxicol Rep. 2022;9:1766-1776.
[19] MAATOUGH A, WHITFIELD GK, BROOK L, et al. Human Hairless Protein Roles in Skin/Hair and Emerging Connections to Brain and Other Cancers. J Cell Biochem. 2018;119(1):69-80.
[20] BROOK L, WHITFIELD GK, HSIEH D, et al. The Mammalian Hairless Protein as a DNA Binding Phosphoprotein. J Cell Biochem. 2017;118(2): 341-350.
[21] LIU L, RODRIGUEZ-MATEO C, HUANG P, et al. Hairless regulates heterochromatin maintenance and muscle stem cell function as a histone demethylase antagonist. Proc Natl Acad Sci U S A. 2021; 118(37):e2025281118.
[22] THOMPSON CC. Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci. 1996;16:7832-7840.
[23] POTTER GB, ZARACH JM, SISK JM et al. The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol. 2002;16(11):2547-2560.
[24] MORAITIS AN, GIGUÈRE V, THOMPSON CC. Novel mechanism of nuclear receptor corepressor interaction dictated by activation function 2 helix determinants. Mol Cell Biol. 2002;22(19):6831-6841.
[25] ENGELHARD A, CHRISTIANO AM. The hairless promoter is differentially regulated by thyroid hormone in keratinocytes and neuroblastoma cells. Exp Dermatol. 2004;13(4):257-264.
[26] THOMPSON CC, BOTTCHER MC. The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci U S A. 1997;94(16):8527-8532.
[27] POTTER GB, BEAUDOIN GM 3RD, DERENZO CL, et al. The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev. 2001;15(20):2687-2701.
[28] 王纯耀,章金涛,祝庆蕃,等.豫医无毛小鼠近交系建立及其生物学特性[J].上海实验动物科学,1999(1):67-68.
[29] ZHANG JT, FANG SG, WANG CY. A novel nonsense mutation and polymorphisms in the mouse hairless gene. J Invest Dermatol. 2005; 124(6):1200-1205.
[30] ZHANG H, WANG S, ZHOU Q, et al. Disturbance of calcium homeostasis and myogenesis caused by TET2 deletion in muscle stem cells. Cell Death Discov. 2022;8(1):236.
[31] CHEN LY, WANG LW, WEN J, et al. RNA-binding protein YBX3 promotes PPARγ-SLC3A2 mediated BCAA metabolism fueling brown adipogenesis and thermogenesis. Mol Metab. 2024;90:102053.
[32] CÓZAR-CASTELLANO I, PERDOMO G. Assessment of Insulin Tolerance In Vivo in Mice. Methods Mol Biol. 2020;2128:217-224.
[33] KENNARD MR, NANDI M, CHAPPLE S, et al. The glucose tolerance test in mice: Sex, drugs and protocol. Diabetes Obes Metab. 2022; 24(11):2241-2252.
[34] MAFFEI M, HALAAS J, RAVUSSIN E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155-1161.
[35] PEREIRA S, CLINE DL, GLAVAS MM, et al. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr Rev. 2021;42(1):1-28.
[36] PICÓ C, PALOU M, POMAR CA, et al. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022;23(1):13-30.
[37] STERN JH, RUTKOWSKI JM, SCHERER PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016;23(5):770-784.
[38] LI X, ZHANG D, VATNER DF, et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci U S A. 2020;117(51):32584-32593.
[39] TANG C, ZHAO H, KONG L, et al. Probiotic Yogurt Alleviates High-Fat Diet-Induced Lipid Accumulation and Insulin Resistance in Mice via the Adiponectin Pathway. J Agric Food Chem. 2023;71(3):1464-1476.
[40] BIANCO AC, SILVA JE. Nuclear 3,5,3’-triiodothyronine (T3) in brown adipose tissue: receptor occupancy and sources of T3 as determined by in vivo techniques. Endocrinology. 1987;120(1):55-62.
[41] BRÉLIVET Y, ROCHEL N, MORAS D. Structural analysis of nuclear receptors: from isolated domains to integral proteins. Mol Cell Endocrinol. 2012;348(2):466-473.
[42] MUKHERJEE S, DASGUPTA S, PANJA SS, et al. Structural insight to human Retinoid X receptor alpha-Thyroid hormone receptor beta heterodimer by molecular modelling and MD-simulation studies: role of conserved water molecules. J Biomol Struct Dyn. 2023;41(19):9828-9839.
[43] MANGELSDORF DJ, EVANS RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841-850.
[44] TAMBONES I, LE MAIRE A. Structural Insights Into Thyroid Hormone Receptors. Endocrinology. 2024;166(1):bqae154.
[45] ASTAPOVA I, HOLLENBERG AN. The in vivo role of nuclear receptor corepressors in thyroid hormone action. Biochim Biophys Acta. 2013; 1830(7):3876-3881.
[46] DJABALI K, CHRISTIANO AM. Hairless contains a novel nuclear matrix targeting signal and associates with histone deacetylase 3 in nuclear speckles. Differentiation. 2004;72(8):410-418.
[47] SØBERG S, LÖFGREN J, PHILIPSEN FE, et al. Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men. Cell Rep Med. 2021;2(10):100408.
[48] BAGON BB, LEE J, MATIENZO ME, et al. Cold-induced adaptive thermogenesis is impaired by exposure of Asian sand dust in mice. J Therm Biol. 2023;116:103675.
[49] BRTKO J. Thyroid hormone and thyroid hormone nuclear receptors: History and present state of art. Endocr Regul. 2021;55(2):103-119.
[50] PUIGSERVER P, WU Z, PARK CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998; 92(6):829-839.
[51] MIRANDA CS, SILVA-VEIGA FM, SANTANA-OLIVEIRA DA, et al. PPARα/γ synergism activates UCP1-dependent and -independent thermogenesis and improves mitochondrial dynamics in the beige adipocytes of high-fat fed mice. Nutrition. 2024;117:112253.
[52] SHIBATA Y, EGUCHI J, WADA J. Brown Adipose Tissue PPARγ Is Required for the Insulin-Sensitizing Action of Thiazolidinediones. Acta Med Okayama. 2023;77(3):243-254.
[53] SOLMONSON A, MILLS EM. Uncoupling Proteins and the Molecular Mechanisms of Thyroid Thermogenesis. Endocrinology. 2016;157(2): 455-462.
[54] LIU S, SHEN S, YAN Y, et al. Triiodothyronine (T3) promotes brown fat hyperplasia via thyroid hormone receptor α mediated adipocyte progenitor cell proliferation. Nat Commun. 2022;13(1):3394.
[55] YAU WW, SINGH BK, LESMANA R, et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy. 2019;15(1):131-150.
[56] WU Z, PUIGSERVER P, ANDERSSON U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115-124.
[57] PILLAR TM, SEITZ HJ. Thyroid hormone and gene expression in the regulation of mitochondrial respiratory function. Eur J Endocrinol. 1997;136(3):231-239.
|