中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (10): 2536-2549.doi: 10.12307/2026.644
• 组织构建综述 tissue construction review • 上一篇 下一篇
胶质-神经元互作在基底节退行性疾病中的调控机制及潜在治疗靶点
- 1北华大学基础医学院人体解剖学教研室,吉林省吉林市 132013;2北华大学附属医院生殖中心,吉林省吉林市 132013;3吉林市中心医院重症二科,吉林省吉林市 132013
-
收稿日期:2025-05-21接受日期:2025-06-20出版日期:2026-04-08发布日期:2025-08-29 -
通讯作者:王鹏,博士,教授,硕士生导师,北华大学基础医学院人体解剖学教研室,吉林省吉林市 132013 -
作者简介:李明徽,女,1989年生,吉林省人,蒙古族,北华大学基础医学院在读硕士,主要从事帕金森病机制方面的研究。 -
基金资助:吉林省科技厅科技发展计划项目(20240402007GH),项目负责人,王鹏
Glial-neuronal interactions in basal ganglia neurodegenerative diseases: regulatory mechanisms and potential therapeutic targets
Li Minghui1, 2, Zhang Yingbi1, 2, Zhang Xiaorui1, Yin Jihong1, 3, Wang Peng1
- 1Department of Human Anatomy, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China; 2Reproductive Center of Beihua University Affiliated Hospital, Jilin 132013, Jilin Province, China; 3The Second Intensive Care Unit, Jilin Central Hospital, Jilin 132013, Jilin Province, China
-
Received:2025-05-21Accepted:2025-06-20Online:2026-04-08Published:2025-08-29 -
Contact:Wang Peng, PhD, Professor, Master’s supervisor, Department of Human Anatomy, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China -
About author:Li Minghui, MS candidate, Department of Human Anatomy, School of Basic Medical Sciences, Beihua University, Jilin 132013, Jilin Province, China; Reproductive Center of Beihua University Affiliated Hospital, Jilin 132013, Jilin Province, China -
Supported by:Science and Technology Development Program of Jilin Provincial Department of Science and Technology, No. 20240402007GH (to WP)
摘要:
文题释义:
胶质-神经元互作:是指神经元与神经胶质细胞(包括星形胶质细胞、小胶质细胞和少突胶质细胞等)间的双向信号交流与功能调控。这种相互作用在支持神经元代谢、突触可塑性、免疫监视及神经环路稳态中发挥关键作用。胶质-神经元互作的失调参与多种神经系统疾病的发生和发展。
基底节退行性疾病:是一类以基底神经节神经元进行性功能障碍为主要特征的神经退行性疾病,包括帕金森病、亨廷顿病和多系统萎缩等。这些疾病通常以运动功能障碍(如运动迟缓、肌张力异常)以及认知和情绪障碍为主要表现,其病理机制复杂,涉及神经元代谢失衡、神经系统炎症和突触功能障碍等因素。
背景:胶质-神经元互作通过代谢支持、免疫调节和突触修剪等功能维持中枢神经系统稳态,其功能异常与基底节退行性疾病的病理进程密切相关。
目的:总结胶质-神经元互作在基底节退行性疾病中的调控机制与作用,并探讨其潜在的治疗靶点。
方法:检索中国知网、PubMed和Web of Science数据库2020年1月至2024年11月发表的相关文献(除经典文献外)。英文检索词:basal ganglia,striatum,substantia nigra,globus pallidus;glial cells,astrocytes,microglia,oligodendrocytes;neurons,neurodegeneration,synaptic dysfunction;Parkinson’s disease,Huntington’s disease,multiple system atrophy,neurodegenerative diseases;metabolic coupling,neuroinflammation,synaptic pruning,oxidative stress,organoid model,single-cell sequencing;therapeutic targets,glial modulation,neuroprotection,blood-brain barrier,off-target effect;中文检索词:基底节,纹状体,黑质,苍白球;胶质细胞,星形胶质细胞,小胶质细胞,少突胶质细胞;神经元,神经退行性变,突触功能障碍;帕金森病,亨廷顿病,多系统萎缩,神经退行性疾病;代谢耦合,神经炎症,突触修剪,氧化应激,类器官模型,单细胞测序;治疗靶点,胶质细胞调控,神经保护,血脑屏障,脱靶效应。根据纳入和排除标准,最终筛选出113篇文献进行归纳总结。
结果与结论:①胶质-神经元互作的失调导致代谢失衡、神经炎症和突触功能障碍,进而加剧基底节神经元的退行性变;②在帕金森病中,星形胶质细胞谷氨酸转运体功能受损,导致谷氨酸清除能力下降,引发神经元毒性;③在多系统萎缩中,少突胶质细胞的功能障碍导致神经元脱髓鞘和轴突损伤;④在亨廷顿病中,小胶质细胞通过Toll样受体4介导细胞因子释放,加剧神经炎症;⑤靶向胶质-神经元互作的治疗策略(如大麻素受体2受体激动剂、NOD样受体热蛋白结构域相关蛋白3抑制剂等)在动物实验和临床试验中均展现出潜力;⑥胶质-神经元互作在基底节退行性疾病的病理进程中发挥重要作用,针对于此的靶向治疗策略在基底节退行性疾病中有广阔的治疗前景。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
李明徽, 张瑛碧, 张晓瑞, 殷继红, 王 鹏 . 胶质-神经元互作在基底节退行性疾病中的调控机制及潜在治疗靶点[J]. 中国组织工程研究, 2026, 30(10): 2536-2549.
Li Minghui, Zhang Yingbi, Zhang Xiaorui, Yin Jihong, Wang Peng. Glial-neuronal interactions in basal ganglia neurodegenerative diseases: regulatory mechanisms and potential therapeutic targets[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2536-2549.
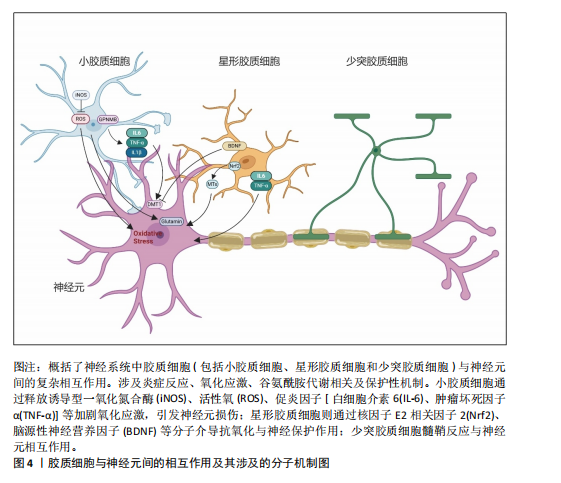
2.2 胶质细胞-神经元互作在基底节退行性疾病中的作用机制 胶质细胞在神经炎症过程中扮演了“双刃剑”的角色:在正常生理范畴,它们发挥正性作用,参与突触的可塑性,促进神经环路的发育,对维持中枢神经系统正常的神经元功能至关重要[13]。星形胶质细胞及小胶质细胞可与神经元突触之间形成较为密切的直接接触[31],还可通过释放细胞因子和可溶性因子,如脑源性神经营养因子、谷氨酸、肿瘤坏死因子α、白细胞介素1β、甘氨酸和L-丝氨酸等,直接作用于神经元,影响基础神经传递和突触可塑性[32]。星形胶质细胞除了谷氨酸外,还可以分泌其他递质,如γ-氨基丁酸、ATP和D-丝氨酸等,通过与神经元的相应受体结合,调节突触传递和可塑性[33]。这些过程使大脑依赖于不间断的能量供应,H?SLI等[34]发现星形胶质细胞与脉管系统相连,维持糖酵解功能,维持自身需求并调节对神经元和突触的能量,当能量供应失败时,脉管系统衰竭或星形胶质细胞病理学,大脑代谢和随后的神经传递处于危险之中。另外神经元释放的谷氨酸被星形胶质细胞摄取,转化为谷氨酰胺后作用神经元,可调控突触活动能量需求。因此支持代谢功能至关重要[35]。激活的M1小胶质细胞能量消耗依赖糖酵解,过程中产生大量乳酸和活性氧,这些产物又加剧神经元氧化应激[36]。神经元-胶质互作通过动态能量供应、代谢废物清除和线粒体支持维持脑能量稳态,其失衡是神经退行性疾病的核心病理环节,可见神经元与胶质细胞之间以及胶质细胞之间的双向通信在大脑功能中起着关键作用,见图4。最近的研究表明,异常的神经元-胶质相互作用存在于大多数已知的中枢神经病理状况,包括帕金森病、亨廷顿病、多系统萎缩以及神经发育障碍如自闭症谱系障碍和抑郁症等[37]。
2.2.1 胶质-神经元互作在帕金森病中的作用机制 帕金森病是仅次于阿尔茨海默病的第2大常见神经退行性疾病,其临床症状包括运动症状(如
运动迟缓、静止性震颤、僵硬和姿势不稳)和非运动症状(如嗅觉减退、便秘、睡眠障碍和抑郁)。在欧洲,帕金森病的患病率和发病率分别为(108-257)/10万和(11-19)/10万[38]。截至2021年国内帕金森病的发病率、患病率和死亡率分别为35.73/10万、356.85/10万、6.47/10万[39]。帕金森病的主要病理改变特征是黑质致密部多巴胺能神经元的丢失以及路易体中错误折叠的α-突触核蛋白累积。α-突触核蛋白在体内以无毒且可溶的单体形式存在,但其聚集会导致毒性功能的获得[40]。研究表明多种遗传和环境因素调节α-突触核蛋白单体向聚集体转化,这一过程涉及多种细胞参与[41]。帕金森病的病因尚不完全清楚,但研究表明遗传因素(PARK等基因突变)以及污染物、农药、重金属和感染等环境因素可能与患病风险增加相关[42]。
帕金森病中,小胶质细胞的异常激活及其与神经元的动态互作是驱动神经炎症与多巴胺能神经元退变的核心机制。早期病理研究已证实,帕金森病患者黑质区小胶质细胞呈现持续性激活状态,表现为促炎标记物诱导型一氧化氮合酶和吞噬表型标记物CD68的显著上调[43-45]。单核RNA测序进一步揭示了帕金森病相关小胶质细胞的异质性:黑质纹状体通路中,嘌呤能受体(P2RY12)高表达的稳态亚群逐渐分化为热休克蛋白90KDaα(胞浆),类A成员1(HSP90AA1/IL1B)或重组人非转移性行黑色素瘤糖蛋白B主导的激活亚群,提示不同功能状态的小胶质细胞可能通过时空特异性方式参与疾病进程[46]。此外,α-突触核蛋白聚集体不仅直接激活小胶质细胞炎症小体,还可作为趋化信号引导其向受损神经元迁移[47],这一过程伴随白细胞介素1β、肿瘤坏死因子α等细胞因子的释放,并通过外周循环募集单核细胞浸润中枢神经系统[48-52]。
在调控层面,小胶质细胞的激活状态受到多重信号通路的精细平衡。抑制性信号轴包括:①趋化因子C-X3-C-基元配体1/趋化因子C-X3-C-基元受体1(CX3CL1-CX3CR1)信号通路:小胶质细胞表达的趋化因子CX3CR1受体通过结合神经元分泌的CX3CL1配体,抑制其过度激活并维持突触可塑性[53]。动物实验表明,纹状体持续输注CX3CL1可显著减轻6-羟多巴胺模型中的小胶质细胞活化与多巴胺能神经元丢失[54];②CD200-CD200R通路:神经元表面CD200与小胶质细胞CD200R的相互作用,对维持小胶质细胞的静息状态至关重要。临床研究显示,帕金森病患者外周来源的巨噬细胞中CD200R的表达水平显著降低,提示该免疫调节通路可能存在功能缺陷。动物实验中,阻断CD200-CD200R结合(如纹状体注射CD200R抗体)可加重6-羟多巴胺模型大鼠的运动障碍,减少黑质多巴胺能神经元数量,并伴随小胶质细胞异常活化及促炎因子释放。这表明CD200-CD200R系统功能缺陷会解除对小胶质细胞的抑制,加速神经元退行性变[55]。促进性信号轴则以CC趋化因子配体2(CC chemokine ligand 2,CCL2)/CC趋化因子受体2(CC chemokine
receptor,CCR2)通路为代表:α-突触核蛋白异常聚集诱导CCL2分泌,通过激活外周单核细胞CCR2受体促进其向中枢神经系统浸润,这一过程与帕金森病患者血清CCL2水平及疾病亚型显著相关[52]。此外,多项帕金森动物模型表明,内源性大麻素系统通过小胶质细胞大麻素受体2的激活发挥神经保护作用,而基质金属蛋白酶2/基质金属蛋白酶9的异常活化可能破坏血脑屏障完整性,形成神经炎症的正反馈环路[56-58]。
在帕金森病的病理进程中,胶质细胞与神经元间交互网络的失衡形成了自我强化的恶性循环:过度激活的小胶质细胞释放白细胞介素6、肿瘤坏死因子α等促炎因子,不仅直接诱导多巴胺能神经元损伤,还会通过抑制星形胶质细胞谷氨酸转运体谷氨酸转运体1的功能,导致突触间隙谷氨酸清除障碍及兴奋性毒性[59-60]。这种神经炎症与神经退行性变的相互促进,提示通过靶向调控关键通路(如增强CX3CL1/CD200抑制性信号或阻断CCL2/基质金属蛋白酶促炎通路)可能重塑胶质-神经元稳态,从而为干预帕金森病的疾病进展提供新策略。
2.2.2 胶质-神经元互作在多系统萎缩中的作用机制 多系统萎缩是一种罕见、进行性且致命的神经退行性疾病,其特征包括自主神经功能障碍、帕金森综合征和小脑性共济失调,其发病率估计为(0.6-0.7)/10万[7]。根据主要症状,多系统萎缩在临床上分为2种亚型:以橄榄桥小脑萎缩引起小脑性共济失调为主的多系统萎缩-C型,以纹状体黑质变性导致帕金森综合征为主的多系统萎缩-P型[61]。多系统萎缩的病理标志是少突胶质细胞胞质中α-突触核蛋白的异常积累,称为胶质细胞质包涵体。从机制上看,多种因素参与了多系统萎缩的发病机制,包括α-突触核蛋白的异常积累、小胶质细胞激活和神经炎症、自噬功能障碍、线粒体功能障碍以及蛋白酶体功能障碍[62]。其中小胶质细胞激活和神经炎症也是多系统萎缩的重要特征之一[63-65]。研究表明,Toll样受体4(Toll-like receptor 4,TLR4)介导的小胶质细胞对α-突触核蛋白的吞噬作用,诱导核因子κB易位、活性氧生成以及促炎细胞因子(如肿瘤坏死因子α)的释放[66]。α-突触核蛋白激活小胶质细胞还依赖于TLR1/2信号通路,这表明TLRs在α-突触核蛋白诱导的小胶质细胞促炎反应和活性氧释放中具有调节作用[67]。最新研究在多系统萎缩患者的脑组织中发现一些含有α-突触核蛋白的小胶质细胞远离含有胶质细胞质内含物的少突胶质细胞,因此推测这些小胶质细胞在摄取α-突触核蛋白后并未就地降解这些错误折叠的蛋白质,而是作为载体在多系统萎缩中传播α-突触核蛋白[68],这提示特异性抑制运输α-突触核蛋白的胶质细胞迁移可成为潜在的治疗措施。此外,在多系统萎缩患者的死后脑组织和病毒介导的多系统萎缩小鼠模型中,均观察到T细胞浸润伴炎性小胶质细胞增生,纹状体组织中Th1 T细胞的比例和干扰素γ细胞因子的水平显著增加,这证实少突胶质细胞中的α-突触核蛋白可诱导小胶质细胞激活,触发CD4+ T细胞浸润。CD4+ T细胞浸润中枢神经系统后,其T细胞受体与小胶质细胞表面上调的组织相容性复合体Ⅱ结合,促进CD4 T细胞分化为Th1 T细胞并分泌干扰素γ,这一途径导致少突胶质细胞功能障碍以及纹状体和胼胝体脱髓鞘,产生多系统萎缩主要症状[69]。可见T细胞浸润中枢神经系统及其与小胶质细胞的相互作用是多系统萎缩疾病发病机制的关键环节。
2.2.3 胶质细胞-神经元互作在亨廷顿病中的作用机制 亨廷顿病是一种遗传性神经退行性疾病,表现为不自主的舞蹈样动作以及认知和行为障碍。亨廷顿病是一种单基因常染色体显性遗传病,由亨廷顿蛋白基因中CAG三核苷酸重复序列的扩展引起。该突变导致亨廷顿蛋白中出现异常长的多聚谷氨酰胺扩展,使蛋白质易于错误折叠[70]。发达国家人群中亨廷顿病的发病率为(4-10)/10万,平均发病年龄为40岁[71]。致病性突变亨廷顿蛋白在大脑的多种神经元中表达,异常聚集的亨廷顿蛋白通常以包涵体的形式存在于细胞质或细胞核中,导致纹状体中的中型多棘神经元选择性丢失以及尾状核和壳核萎缩[72]。目前普遍认为N端亨廷顿蛋白片段在亨廷顿病发病机制中起重要作用,较小的N端亨廷顿蛋白片段比较大的片段表现出更强的神经毒性[73]。
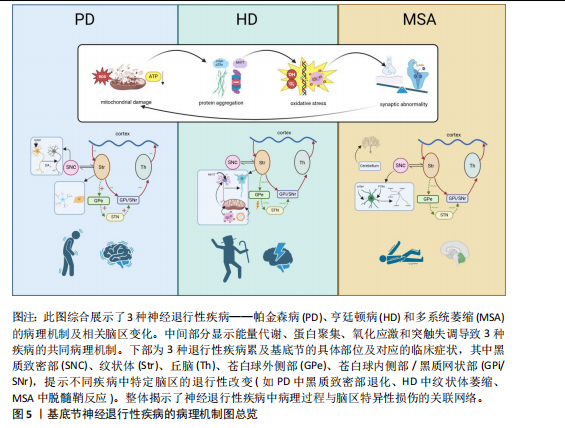
在亨廷顿病患者的纹状体中常发现反应性胶质增生,包括反应性纤维性星形胶质细胞增生和反应性小胶质细胞增生[74]。在皮质-纹状体脑切片和原代神经元培养模型中,神经元中亨廷顿蛋白的表达导致小胶质细胞激活。这些反应性小胶质细胞倾向于聚集在异常神经突起周围,但并不直接导致神经元退化[75]。亨廷顿病中小胶质细胞的激活涉及多种信号通路,包括核因子κB通路等,小胶质细胞检测外部刺激激活后,TLRs触发一系列信号通路,导致免疫反应的激活。TLRs信号通过细胞内适配髓样分化因子88(myeloid differentiation factor 88,MyD88)因子激活下游核因子κB信号级联反应,从而增加促炎细胞因子的产生。研究表明,可溶性亨廷顿蛋白激活IκB激酶,触发核因子κB信号通路,并导致促炎细胞因子基因表达的增加[76]。通过siRNA降低亨廷顿蛋白水平改善了核因子κB转录失调并减少了亨廷顿病中促炎细胞因子的产生[77]。这些发现表明小胶质细胞动态状态的变化可能导致其在亨廷顿病中的神经支持功能受损,见图5。
2.3 胶质-神经元互作技术前沿 胶质神经元互作技术是研究神经系统功能、发育和疾病的重要工具,其中类器官模型和单细胞测序技术的结合极大地推动了这一领域的发展。
2.3.1 类器官模型的贡献 脑类器官技术是通过模拟体内微环境,揭示胶质细胞对神经发育以及细胞间复杂的调控关系。ZHENG等[78]将星形胶质细胞的条件培养基加入到人源多能干细胞衍生的二维和三维神经培养系统中,发现星形胶质细胞分泌的因子可以加速神经元的分化,增强神经元网络的电生理活性,还能通过脂滴介导的应激保护机制,促进脑类器官的功能成熟。PARK等[79]将人诱导多能干细胞衍生的原始样巨噬细胞与脑类器官共培养,在该模型中,原始样巨噬细胞分化为小胶质样细胞,并通过脂肪分化相关蛋白脂滴介导胆固醇及其酯的输出,促进神经祖细胞的脂质摄取,进而调控神经发生过程,抑制细胞增殖并增强轴突形成。这一研究填补了脑类器官中小胶质细胞功能研究的空白,并在小鼠和人胚胎脑中验证了该脂质代谢互作机制的保守性,提示其在神经发生中的普适性。CAKIR等[80]在人胚胎干细胞来源的皮质类器官中过表达髓系转录因子PU.1,构建了含有功能性小胶质细胞的类器官模型,他们发现这些小胶质细胞可以激活补体/趋化因子系统,减轻β-淀粉样蛋白诱导的神经元损伤,抑制凋亡和铁死亡相关基因的表达;此外,他们还利用混合成簇规律间隔的短回文重复序列干扰(clustered regularly interspaced short palindromic repeat interference,CRISPRi)和单细胞转录组技术解析了阿尔茨海默病风险基因的功能,为研究小胶质细胞在神经退行性疾病中的作用提供了新的研究平台。
2.3.2 单细胞测序的贡献 FLECK等[81]通过整合了人神经类器官的单细胞多组学数据,并利用自主开发的Pando框架构建了全局基因调控网络,研究发现,转录因子GLI3通过调控HES4/5,影响背腹模式和神经节隆起的多样化,从而决定了端脑的命运。结合混合遗传扰动实验,进一步验证了转录因子对细胞命运和状态的调控层级,为利用类器官模型解析人类大脑发育的基因调控机制提供了系统性的研究范式。WU等[82]首次发现周围神经系统中存在一种与中枢小胶质细胞具有相似转录组和表观遗传特征的巨噬细胞群(周围神经系统小胶质样细胞),这些细胞通过包裹神经元胞体,调控发育中的胞体增大和轴突生长;系统发育分析表明,这种细胞在脊椎动物中的存续与神经元胞体大小(而非进化距离)密切相关,尤其在大胞体神经元物种中更为常见。这一发现确立了中枢神经系统小胶质细胞的周围神经系统同源细胞,并揭示了它们在神经元形态进化中的调控作用。
2.3.3 类器官与单细胞测序的联合应用 类器官与单细胞测序的联合应用正在为精准医学的发展提供新的思路。VICTOR等[83]利用CRISPR编辑的诱导多能干细胞模型,发现APOE4等位基因会导致小胶质细胞脂质异常蓄积,增强脂肪生成程序,削弱其对神经元活动的响应能力,同时加剧促炎信号传导,最终导致神经元网络协调性受损,这表明阿尔茨海默病患者中神经元功能障碍可能源于小胶质细胞脂质稳态失衡引发的非细胞自主性调控紊乱。LENG等[84]则结合CRISPR干扰筛选和单细胞转录组学技术,揭示了白细胞介素6/干扰素自分泌-旁分泌信号通过STAT3双向调控炎性星形胶质细胞的反应性。这种反应性在阿尔茨海默病等人类脑疾病及小鼠模型中异常表达,为靶向调节神经炎症反应提供了分子机制和治疗策略。
2.4 靶向胶质细胞-神经元互作的治疗策略 胶质细胞的激活及其介导的炎症反应是神经退行性疾病发展中的关键因素,因此理解胶质细胞激活的复杂性和失衡可能为包括帕金森病在内的神经退行性疾病的治疗干预提供新思路。
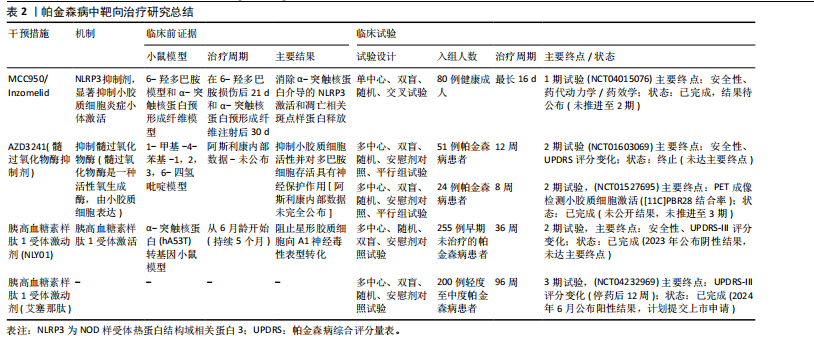
2.4.1 帕金森病相关治疗策略 调节或修饰小胶质细胞受体Toll样受体和大麻素受体2为帕金森病治疗提供了新的药理学方法。通过调节Toll样受体2及其下游信号成分蛋白髓样分化(MyD88)、肿瘤坏死因子受体连接因子6和转化生长因子激酶1可对1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病模型鼠具有保护作用[85]。大麻素受体2的天然激动剂β-石竹烯和选择性大麻素受体2受体激动剂JWH133均在模型小鼠中通过调节小胶质细胞激活和抑制促炎细胞因子的表达保护神经元[86]。这些均表明大麻素受体2受体激动剂可能作为帕金森病的潜在治疗靶点。此外,NLRP3抑制剂MCC950显著抑制了炎症小体激活、α-突触核蛋白聚集体的积累以及黑质纹状体多巴胺能神经元的退化,改善帕金森病模型小鼠的运动功能[87]。研究表明,维生素D通过抑制小胶质细胞激活和促进M2极化保护多巴胺能神经元免受炎症和氧化应激的损害,同时增加M2小胶质细胞标志物CD163、CD204和CD206的表达[88]。过氧化物酶体增殖物激活受体γ激动剂罗格列酮通过调节小胶质细胞极化对多巴胺能神经元发挥神经保护作用。罗格列酮减少促炎细胞因子肿瘤坏死因子α和白细胞介素1β,增加抗炎细胞因子转化生长因子β和白细胞介素10,诱导M2极化,并缓解黑质致密部神经元的多巴胺能神经元变性[89]。北美临床试验注册方案已通过的帕金森病临床试验中的小胶质细胞靶向疗法中,NLRP3抑制剂Inzomelid的1期临床试验招募已于2020年完成(NCT04015076),但结果尚未公布。目前暂无美国食品药品监督管理局审批记录,但2019年获得针对克罗恩病的孤儿药资格。美国国立卫生院资助多项与NLRP3相关炎症小体的研究,其中由约翰霍普金斯大学启动的$3.1 million(2018–2023年)资助项目探讨了NLRP3在帕金森病中的作用(R01NS099876),并发现NLRP3抑制剂可减少α-突触核蛋白诱导的神经元死亡[90]。髓过氧化物酶是小胶质细胞等吞噬细胞产生活性氧的关键酶,也是许多疾病中炎症过程的介质[91]。因此,抑制髓过氧化物酶是减少帕金森病神经炎症的一个有吸引力的靶点。选择性不可逆髓过氧化物酶抑制剂AZD3241在帕金森病患者中通过PET成像评估减少了小胶质细胞激活,并且耐受性良好[92]。美国国立卫生院资助了多项与髓过氧化物酶在神经退行性疾病中作用相关的学术研究,其中由宾夕法尼亚大学启动的$2.3 million(2013–2018年)资助项目“髓过氧化物酶与α-突触核蛋白病理的相互作用(R01NS078507)”发现,髓过氧化物酶介导α-突触核蛋白硝基化,并加速其聚集。2023年美国食品药品监督管理局审批通过Cinpanemab(抗α-突触核蛋白单抗),可以靶向清除神经元或胶质细胞内的α-突触核蛋白[93]。胰高血糖素样肽1受体激动剂通常用于治疗2型糖尿病,胰高血糖素样肽1受体激动剂NLY01在hA53T转基因小鼠中直接阻止小胶质细胞介导的星形胶质细胞向A1神经毒性表型的转化[94]。胰高血糖素样肽1类似物司美格鲁肽和利拉鲁肽在慢性1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病小鼠模型中减少了小胶质细胞激活、α-突触核蛋白积累和多巴胺能神经元的丢失,并改善了运动功能障碍[95]。
2021年美国食品药品监督管理局授予其针对帕金森病的快速通道资格,2022年授予其针对帕金森病的孤儿药资格。美国国立卫生研究院资助了多项与胰高血糖素样肽1受体在神经退行性疾病中作用相关的研究,其中由约翰霍普金斯大学启动了$3.2 million (2018-2023年)资助项目“胰高血糖素样肽1受体激活对α-突触核蛋白病理的影响(R01NS099876)”,胰高血糖素样肽1激动剂可减少α-突触核蛋白聚集并改善小鼠运动功能[96]。艾塞那肽是研究最广泛的胰高血糖素样肽1受体激动剂之一,可改善帕金森病患者运动症状,耐受性良好,其效果在给药后可持续12个月[97]。见表2。
2.4.2 多系统萎缩相关治疗策略 在多系统萎缩小鼠模型的早期应用髓过氧化物酶抑制剂,可抑制小胶质细胞激活和神经炎症,减少α-突触核蛋白的胞内聚集,缓解神经元丢失,改善了运动障碍[98]。但在疾病晚期严重多系统萎缩样神经病理学出现以后给予髓过氧化物酶抑制剂进行治疗观察,虽然可检测到小胶质细胞激活减少和α-突触核蛋白的累积,但神经元变性并未得以控制、运动障碍也并无明显改善。这一结果提示抑制小胶质细胞激活治疗时机选择的重要性,
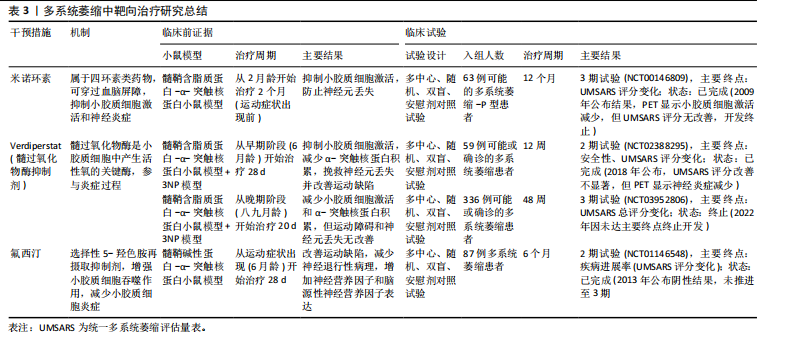
尽早干预小胶质细胞激活是控制疾病进展的重要条件[99]。北美临床试验注册方案已通过:Verdiperstat(髓过氧化物酶抑制剂)已在约250名健康志愿者和患者中进行了1期和2期临床试验。针对多系统萎缩患者的2期试验结果显示,髓过氧化物酶抑制剂治疗12周后,临床评分有所改善,PET成像显示神经炎症减少(NCT02388295)。正在进行中的3期研究以评估Verdiperstat(BHV-3241)治疗48周对多系统萎缩患者的疗效和安全性,其中使用统一多系统萎缩评估量表(Unified Multiple System Atrophy Rating Scale,UMSARS)评分评估verdiperstat的疗效(NCT03952806)。有些靶点抑制在动物模型中虽然效果较好,但临床试验失败。2018年美国食品药品监督管理局针对多系统萎缩适应证授予孤儿药资格,2019年授予快速通道资格,但因Ⅱ/Ⅲ期试验失败,Biohaven终止开发,未向美国食品药品监督管理局提交新药申请。美国国立卫生院资助了多项与髓过氧化物酶在神经退行性疾病中作用相关的研究:其中包括由梅奥诊所启动$2.5 million(2020–2025年)资助项目“髓过氧化物酶介导的氧化损伤在多系统萎缩中的作用(R01NS112345)”,发现髓过氧化物酶活性与少突胶质细胞质内含物密度正相关[100]。如米诺环素,能够穿过血脑屏障并抑制小胶质细胞激活和促炎细胞因子的产生,在转基因多系统萎缩小鼠模型中应用,抑制小胶质细胞激活并防止黑质致密部多巴胺能神经元和纹状体多巴胺能末梢的丢失[101-102]。其后开展的临床试验以评估米诺环素对63例多系统萎缩-P型患者的疗效(NCT00146809),经过12个月的米诺环素治疗,PET成像显示小胶质细胞虽激活减少,但患者运动功能未有明显改善[103]。美国国立卫生院资助了多项与米诺环素神经保护作用相关的基础研究:其中包括埃默里大学启动$0.4 million(2014-2017年)资助米诺环素对帕金森病模型中小胶质细胞极化的影响(R21NS089667),主要评估米诺环素对α-突触核蛋白炎症反应的调控。再如选择性5-羟色胺再摄取抑制剂氟西汀,显著减少脂多糖诱导的炎细胞因子产生和氧化应激,增强了小胶质细胞的吞噬和自噬功能,改善多系统萎缩模型小鼠的运动障碍并减少神经退行性病理的形成[104]。针对多系统萎缩患者的2期双盲、安慰剂对照临床试验研究发现,多系统萎缩患者的进展与对照组相比无差异(NCT01146548)[105]。见表3。
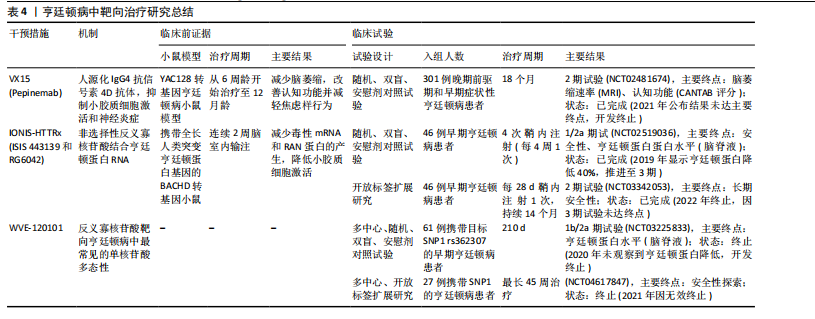
2.4.3 亨廷顿病相关治疗策略 对于亨廷顿病目前尚无特效疗法,现有治疗主要用于缓解舞蹈症和精神症状。鉴于亨廷顿病中的小胶质细胞激活是由神经元亨廷顿蛋白介导的兴奋毒性或小胶质细胞中亨廷顿蛋白表达引起[106],多种治疗方法均以亨廷顿蛋白为靶点,通过减少突变亨廷顿RNA (Htt RNA)的表达,减轻小胶质细胞激活、缓解炎症过程并提供神经保护作用。北美临床试验注册方案已通过:Pepinemab靶向信号素4D的单克隆抗体,抑制信号素4D介导的神经炎症和胶质细胞活化,促进神经元保护和突触可塑性(NCT02481674)[107]。结果显示:可以减少脑萎缩,改善认知功能并减轻焦虑样行为。2021年针对亨廷顿病获批孤儿药资格2022年获美国食品药品监督管理局快速通道资格。目前2期试验已完成,结果未公布。美国国立卫生院资助了多项与信号素4D机制相关的研究,包括加州大学旧金山分校启动$3.5 million(2018–2023年)资助项目“信号素4D在神经炎症中的作用(R01NS099876)”,结果发现抑制信号素4D可减少小胶质细胞活化并改善阿尔茨海默病模型认知功能。反义寡核苷酸——WVE-120101和WVE-120102,靶向亨廷顿病患者中最常见的单核苷酸多态性,已在人体研究中进行了评估(NCT04617847,NCT04617860),结果显示患者脑脊液中的亨廷顿蛋白水平无显著变化。靶向亨廷顿蛋白的反义寡核苷酸 IONIS-HTTRx(也称为ISIS 443139和RG6042)通过RNase H1机制发挥作用,已完成1/2a期临床试
验(NCT02519036,NCT03342053),目前正在进行大型多中心全球疗效研究。阶段性结果显示患者脑脊液中亨廷顿蛋白浓度随给药剂量增加有明显降低,尤其高剂量下的亨廷顿蛋白减少约40%,在安全性上未报告显著不良反应,这为亨廷顿病的治疗带来了希望[108]。见表4。
2.4.4 胶质细胞靶向治疗的挑战 胶质细胞(如小胶质细胞、星形胶质细胞)靶向治疗在神经退行性疾病中展现出潜力,但其临床应用仍面临多重挑战,主要包括血脑屏障穿透效率不足、脱靶效应引发的非特异性毒性,以及胶质细胞功能异质性导致的精准调控难题。以下结合最新研究进展分述关键问题与解决策略:(1)血脑屏障递送障碍:血脑屏障是由脑微血管内皮细胞、星形胶质细胞、周细胞和基膜组成的,它能挡住98%以上的小分子药物和几乎所有大分子(比如抗体、基因编辑工具)进入大脑。虽然传统脂溶性药物可以被动扩散进去,但大多数胶质细胞靶向治疗还是需要主动转运。近年递送技术创新聚焦以下方向:①纳米载体系统:脂质体、聚合物纳米颗粒通过表面修饰(如转铁蛋白受体抗体)可增强血脑屏障穿透性。例如,LI团队[109]在2023年一项研究开发了载有小干扰RNA(small interfering RNA,siRNA)的聚乳酸-羟基乙酸纳米颗粒,通过靶向低密度脂蛋白受体相关蛋白1,成功将siRNA递送至阿尔茨海默病模型小鼠的小胶质细胞,显著降低促炎因子表达;②聚焦超声联合微泡:聚焦超声与微泡相结合,可以局部和瞬时打开血脑屏障,提高药物局部浓度,为药物通过 血脑屏障 输送到大脑提供了一种潜在的策略。GASCA-SALAS等[110]通过一项前瞻性单臂Ⅰ期临床试验(NCT03608553)证实,MR引导聚焦超声(MRgFUS)联合微泡靶向开放右侧顶枕颞叶皮质血脑屏障在帕金森病伴痴呆患者中具有安全性和可重复性,10次治疗中8次通过钆增强显影成功实现血脑屏障可逆开放且无严重不良反应,尽管淀粉样蛋白负荷与脑代谢未见显著变化,但观察到轻度认知改善,为神经退行性疾病中血脑屏障靶向药物递送策略的临床应用提供了可行性依据。
(2)脱靶效应与细胞特异性调控:胶质细胞在中枢神经系统里分布很广,功能也很复杂,所以靶向治疗的时候一定要避免误伤正常细胞。PROFACI等[111]之前用集落刺激因子1受体抑制剂PLX5622来耗竭小胶质细胞,发现虽然小胶质细胞和脑内皮细胞直接接触,但它对健康状态下血脑屏障的结构完整性、功能维持和基因表达其实并不是必需的。不过,PLX5622处理还是会干扰脑内皮细胞的胆固醇代谢,这说明药物对脑血管系统有脱靶效应,也揭示了小胶质细胞和内皮细胞互作的非依赖性以及药理学干预的潜在非特异性影响。这几年的解决方案主要有以下2个方向:①细胞外囊泡:YAO等[112]通过设计RNA适配体(com)与适体结合蛋白(Com-CD63融合蛋白)的特异性互作机制,将Cas9或腺嘌呤碱基编辑器核糖核蛋白主动富集至细胞外囊泡中,构建了高效、瞬时且可多重编辑的递送系统,该系统支持多基因靶点、跨物种Cas9协同应用及体内活性验证,显著提升基因组编辑效率并降低脱靶风险,为基于细胞外囊泡的CRISPR治疗提供了安全可控的技术路径;②细胞类型特异性启动子:SERRANO等[113]基于人IBA1启动子466 bp片段构建腺相关病毒载体,结合miR124靶序列调控,实现了静息态与反应性小胶质细胞的高特异性靶向(转导细胞中95%为小胶质细胞),为解析小胶质细胞功能及开发其靶向基因治疗提供了高效、精准的递送工具。
虽然胶质细胞靶向治疗在神经疾病干预中很有前景,但目前还是受到血脑屏障递送效率、细胞异质性导致的脱靶风险以及胶质细胞和神经元互作复杂调控网络的限制。不过,最近纳米载体、表观遗传编辑和类器官-芯片模型等技术的突破,为解决这些问题提供了新的思路。未来还需要更深入地研究胶质细胞亚群的功能动态
性,并开发时空特异性的递送工具。通过跨学科整合(比如单细胞多组学、生物工程和人工智能),靶向策略会从“广谱抑制”转向“按需调控”,最终释放胶质细胞在神经疾病治疗中的全部潜力。
| [1] DI TELLA S, ZINZI P, ANZUINO I, et al. Social cognition in basal ganglia pathologies: Theory of Mind in Huntington’s and Parkinson’s diseases. Soc Cogn Affect Neurosci. 2025;20(1):nsaf007. [2] YIN Z, YUAN T, YANG A, et al. Contribution of basal ganglia activity to REM sleep disorder in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2024;95(10):947-955. [3] RICCI C. Neurodegenerative Disease: From Molecular Basis to Therapy, 2nd Edition. Int J Mol Sci. 2024;25(2):967. [4] HUANG Y, ZHANG G, LI S, et al. Innate and adaptive immunity in neurodegenerative disease. Cell Mol Life Sci. 2025;82(1):68. [5] HAN CZ. All Fired Up: Microglial Neuroinflammation in Parkinson’s Disease. Biol Psychiatry. 2025;97(7):669-671. [6] CEPEDA C, TONG XP. Huntington’s disease: From basic science to therapeutics. CNS Neurosci Ther. 2018;24(4):247-249. [7] POEWE W, STANKOVIC I, HALLIDAY G, et al. Multiple system atrophy. Nat Rev Dis Primers. 2022;8(1):56. [8] PRANGE SE, BHAKTA IN, SYSOEVA D, et al. Dendrite injury triggers neuroprotection in Drosophila models of neurodegenerative disease. Sci Rep. 2024;14(1):24766. [9] ADAMCZYK A. Glial-neuronal interactions in neurological disorders: molecular mechanisms and potential points for intervention. Int J Mol Sci. 2023;24(7):6274. [10] 蔡星宇, 杨道锋, 卓雪瑞, 等. 人参皂苷Rg3对脂多糖诱导的胶质细胞-神经元互作损伤模型的保护作用[J]. 四川大学学报(医学版),2024,55(6):1543-1549. [11] 苏一洵, 李晖, 易陈菊. 运用化学遗传学方法鉴定星形胶质细胞调控抑制性突触形成的关键蛋白[J]. 神经损伤与功能重建,2022,17(1):1-3. [12] GARCÍA-CÁCERES C, BALLAND E, PREVOT V, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22(1):7-14. [13] ALLEN NJ, LYONS DA. Glia as architects of central nervous system formation and function. Science. 2018;362(6411):181-185. [14] KIRKLEY KS, POPICHAK KA, AFZALI MF, et al. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14(1):99. [15] ZHAO XF, HUFFMAN LD, HAFNER H, et al. The injured sciatic nerve atlas (iSNAT), insights into the cellular and molecular basis of neural tissue degeneration and regeneration. Elife. 2022;11:e80881. [16] LANCASTER MA, KNOBLICH JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194): 1247125. [17] IBRAHIM WW, SKALICKA-WOŹNIAK K, BUDZYŃSKA B, et al. NLRP3 inflammasome inhibition and M1-to-M2 microglial polarization shifting via scoparone-inhibited TLR4 axis in ovariectomy/D-galactose Alzheimer’s disease rat model. Int Immunopharmacol. 2023;119:110239. [18] ROSENBERG RN, IVY N, KIRKPATRICK J, et al. Joseph disease and Huntington disease: protein patterns in fibroblasts and brain. Neurology. 1981;31(8):1003-1014. [19] AHMAD SR, ZEYAULLAH M, ALSHAHRANI AM, et al. Deciphering the enigma of neuron-glial interactions in neurological disorders. Front Biosci (Landmark Ed). 2024;29(4):142. [20] RANSOM BR, ORKAND RK. Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci. 1996;19(8):352-358. [21] XIA MQ, QIN SX, WU LJ, et al. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am J Pathol. 1998;153(1): 31-37. [22] NISHIYAMA H, KNOPFEL T, ENDO S, et al. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99(6):4037-4042. [23] KNAPP DJ, WHITMAN BA, WILLS TA, et al. Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain Behav Immun. 2011;25(Suppl 1):S146-S154. [24] HENEKA MT, CARSON MJ, EL KHOURY J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388-405. [25] ZHOU Z, OKAMOTO K, ONODERA J, et al. Astrocytic cAMP modulates memory via synaptic plasticity. Proc Natl Acad Sci U S A. 2021;118(3):e2016584118. [26] LUKIW WJ, POGUE AI. Vesicular transport of encapsulated microRNA between glial and neuronal cells. Int J Mol Sci. 2020; 21(14):5078. [27] ORR N, STEINMAN L. Epstein-Barr virus and the immune microenvironment in multiple sclerosis: insights from high-dimensional brain tissue imaging. Proc Natl Acad Sci U S A. 2025;122(11):e2425670122. [28] CORTY MM, FREEMAN MR. Cell biology in neuroscience: architects in neural circuit design: glia control neuron numbers and connectivity. J Cell Biol. 2013;203(3):395-405. [29] ALLEN N, EROGLU C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96(3):697-708. [30] VEIGA-FERNANDES H, ARTIS D. Neuronal-immune system cross-talk in homeostasis. Science. 2018;359(6383):1465-1466. [31] PEREA G, NAVARRETE M, ARAQUE A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421-431. [32] HARADA K, KAMIYA T, TSUBOI T. Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain. Front Neurosci. 2015;9:499. [33] LAL R, SINGH A, WATTS S, et al. Experimental models of Parkinson’s disease: challenges and opportunities. Eur J Pharmacol. 2024; 980:176819. [34] HÖSLI L, ZUEND M, BREDELL G, et al. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 2022;39(1):1-9. [35] 熊伟杰, 易陈菊. 胆碱能系统功能障碍对神经退行性疾病的影响[J]. 神经损伤与功能重建,2025,20(3):156-161. [36] 张文滨, 汪永杰, 令垚, 等. 1990-2021年中国帕金森病疾病负担分析和预测[J]. 中华疾病控制杂志,2025,29(1):74-81. [37] ZHENG X, ZHANG H, ZHANG Y, et al. Salidroside ameliorates cerebral ischemic injury and regulates the glutamate metabolism pathway in astrocytes. Front Pharmacol. 2024;15:1472100. [38] SIMANENKOVA A, FUKS O, TIMKINA N, et al. Microglia involvement into acute and chronic brain damage in diabetic rats: impact of GLP-1RA and SGLT-2i. Front Biosci (Landmark Ed). 2024;29(7):265. [39] 曹利华, 赵晖, 贺红娟, 等. 神经元-小胶质细胞串话在抑郁症中的作用及中医药的干预探析[J]. 世界科学技术-中医药现代化,2024,26(12):3086-3096. [40] CARCELES-CORDON M, WEINTRAUB D, CHEN-PLOTKIN AS. Cognitive heterogeneity in Parkinson’s disease: a mechanistic view. Neuron. 2023;111(10):1531-1546. [41] WIRDEFELDT K, ADAMI HO, COLE P, et al. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1-S58. [42] DOORN KJ, MOORS T, DRUKARCH B, et al. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun. 2014;2:90. [43] 李冬青, 秦晓红, 米立志. α-突触核蛋白的结构生物学研究[J]. 中国生物化学与分子生物学报,2023,39(4):531-544. [44] GERHARD A, PAVESE N, HATTON G, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006; 21(2): 404-412. [45] LIU Q, LIU Z, XIE W, et al. Single-cell sequencing of the substantia nigra reveals microglial activation in a model of MPTP. Front Aging Neurosci. 2024;16:1390310. [46] SMAJIĆ S, PRADA-MEDINA CA, LANDOULSI Z, et al. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain. 2022;145(3):964-978. [47] BENNETT JP, KEENEY PM, BROHAWN DG. RNA sequencing reveals small and variable contributions of infectious agents to transcriptomes of postmortem nervous tissues from amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson’s disease subjects, and increased expression of genes from disease-activated microglia. Front Neurosci. 2019;13:235. [48] SCHRÖDER JB, PAWLOWSKI M, MEYER ZU HÖRSTE G, et al. Immune cell activation in the cerebrospinal fluid of patients with Parkinson’s disease. Front Neurol. 2018;9: 1081. [49] TENTILLIER N, ETZERODT A, OLESEN MN, et al. Anti-inflammatory modulation of microglia via CD163-targeted glucocorticoids protects dopaminergic neurons in the 6-OHDA Parkinson’s disease model. J Neurosci. 2016;36(36):9375-9390. [50] HALL S, JANELIDZE S, SUROVA Y, et al. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci Rep. 2018;8(1):13276. [51] SHI Q, GUTIERREZ RA, BHAT MA. Microglia, Trem2, and neurodegeneration. Neuroscientist. 2025;31(2):159-176. [52] MEUCCI O, FATATIS A, SIMEN AA, et al. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97(14):8075-8080. [53] 李楠, 赵仁清, 王斌. 运动改善小胶质细胞介导的神经炎症在预防帕金森病的作用机制[J].中国生物化学与分子生报, 2024,40(6):779-787. [54] PABON MM, BACHSTETTER AD, HUDSON CE, et al. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J Neuroinflammation. 2011;8:9. [55] ZHANG S, WANG XJ, TIAN LP, et al. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2011;8:154. [56] PRICE DA, MARTINEZ AA, SEILLIER A, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009; 29(11):2177-2186. [57] CHUNG YC, SHIN WH, BAEK JY, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp Mol Med. 2016;48(1):e205. [58] RUAN Z, ZHANG D, HUANG R, et al. Microglial activation damages dopaminergic neurons through MMP-2/-9-mediated increase of blood-brain barrier permeability in a Parkinson’s disease mouse model. Int J Mol Sci. 2022;23(5):2793. [59] HERRERA MORO CHAO D, KIRCHNER MK, PHAM C, et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 2022;34(10): 1532-1547.e6. [60] BOOTH HDE, WESSELY F, CONNOR-ROBSON N, et al. RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson’s iPSC-derived astrocytes. Neurobiol Dis. 2019;129:56-66. [61] LYOO CH, JEONG Y, RYU YH, et al. Effects of disease duration on the clinical features and brain glucose metabolism in patients with mixed type multiple system atrophy. Brain. 2008;131(Pt 2):438-446. [62] SALVESEN L, WINGE K, BRUDEK T, et al. Neocortical neuronal loss in patients with multiple system atrophy: a stereological study. Cereb Cortex. 2017;27(1):400-410. [63] NYKJAER CH, BRUDEK T, SALVESEN L, et al. Changes in the cell population in brain white matter in multiple system atrophy. Mov Disord. 2017;32(7):1074-1082. [64] 朱琳, 刘军. 多系统萎缩生物标志物的研究进展[J]. 上海交通大学学报(医学版),2020,40(9):1303-1307+1302. [65] KÜBLER D, WÄCHTER T, CABANEL N, et al. Widespread microglial activation in multiple system atrophy. Mov Disord. 2019;34(4):564-568. [66] SHAO QH, YAN WF, ZHANG Z, et al. Nurr1: a vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology. 2019;144:388-399. [67] DANIELE SG, BÉRAUD D, DAVENPORT C, et al. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 2015;8(376):ra45. [68] VALDINOCCI D, GRANT GD, DICKSON TC, et al. Epothilone D inhibits microglia-mediated spread of alpha-synuclein aggregates. Mol Cell Neurosci. 2018;89:80-94. [69] WILLIAMS GP, MARMION DJ, SCHONHOFF AM, et al. T cell infiltration in both human multiple system atrophy and a novel mouse model of the disease. Acta Neuropathol. 2020;139(5):855-874. [70] PAN L, FEIGIN A. Huntington’s disease: new frontiers in therapeutics. Curr Neurol Neurosci Rep. 2021;21(3):10. [71] NITTARI G, ROY P, MARTINELLI I, et al. Rodent models of Huntington’s disease: an overview. Biomedicines. 2023;11(12):3331. [72] VAN DER BURG JM, BJÖRKQVIST M, BRUNDIN P. Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol. 2009;8(8):765-774. [73] 程扬帆, 张斯睿, 商慧芳. 亨廷顿病的诊治进展[J]. 实用医院临床杂志,2024, 21(5):12-16. [74] KRAFT AD, KALTENBACH LS, LO DC, et al. Activated microglia proliferate at neurites of mutant huntingtin-expressing neurons. Neurobiol Aging. 2012;33(3):621.e17-33. [75] KHOSHNAN A, PATTERSON PH. The role of IκB kinase complex in the neurobiology of Huntington’s disease. Neurobiol Dis. 2011; 43(2):305-311. [76] TRÄGER U, ANDRE R, LAHIRI N, et al. HTT-lowering reverses Huntington’s disease immune dysfunction caused by NFκB pathway dysregulation. Brain. 2014;137(Pt 3):819-833. [77] KOO JH, JANG YC, HWANG DJ, et al. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-κB signaling pathway. Neuroscience. 2017;356:102-113. [78] ZHENG H, FENG Y, TANG J, et al. Astrocyte-secreted cues promote neural maturation and augment activity in human forebrain organoids. Nat Commun. 2025;16:2845. [79] PARK DS, KOZAKI T, TIWARI SK, et al. iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature. 2023;623:397-405. [80] CAKIR B, TANAKA Y, KIRAL FR, et al. Expression of the transcription factor PU.1 induces the generation of microglia-like cells in human cortical organoids. Nat Commun. 2022;13:430. [81] FLECK JS, JANSEN SMJ, WOLLNY D, et al. Inferring and perturbing cell fate regulomes in human brain organoids. Nature. 2023; 621:365-372. [82] WU Z, WANG Y, CHEN WW, et al. Peripheral nervous system microglia-like cells regulate neuronal soma size throughout evolution. Cell. 2025;188:2159-2174.e15. [83] VICTOR MB, LEARY N, LUNA X, et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell. 2022;29:1197-1212.e8. [84] LENG K, ROSE IVL, KIM H, et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat Neurosci. 2022;25: 1528-1542. [85] OJHA S, JAVED H, AZIMULLAH S, et al. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem. 2016;418(1-2):59-70. [86] CALVELLO R, CIANCIULLI A, NICOLARDI G, et al. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol. 2017;12(2):327-339. [87] PISANU A, LECCA D, MULAS G, et al. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTP mouse model of progressive Parkinson’s disease. Neurobiol Dis. 2014;71:280-291. [88] COLL RC, ROBERTSON AA, CHAE JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015; 21(3):248-255. [89] ARATANI Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47-52. [90] JUCAITE A, SVENNINGSSON P, RINNE JO, et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson’s disease. Brain. 2015;138(Pt 9): 2687-2700. [91] PANICKER N, KAM TI, WANG H, et al. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron. 2022;110(15):2422-2437. [92] ZHANG L, ZHANG L, LI L, et al. Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of Parkinson’s disease. J Parkinsons Dis. 2019;9(1):157-171. [93] AVILES-OLMOS I, DICKSON J, KEFALOPOULOU Z, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337-344. [94] MCFARTHING K, BUFF S, RAFALOFF G, et al. Parkinson’s disease drug therapies in the clinical trial pipeline: 2023 update. J Parkinsons Dis. 2023;13(4):427-439. [95] KOPP KO, GLOTFELTY EJ, LI Y, et al. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186:106550. [96] STEFANOVA N, GEORGIEVSKA B, ERIKSSON H, et al. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21(4):393-404. [97] ZHAO X, WANG M, WEN Z, et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne). 2021; 12:721135. [98] KAINDLSTORFER C, SOMMER P, GEORGIEVSKA B, et al. Failure of neuroprotection despite microglial suppression by delayed-start myeloperoxidase inhibition in a model of advanced multiple system atrophy: clinical implications. Neurotox Res. 2015;28(3): 185-194. [99] STEFANOVA N, REINDL M, NEUMANN M, et al. Microglial activation mediates neurodegeneration related to oligodendroglial α-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22(15):2196-2203. [100] DODEL R, SPOTTKE A, GERHARD A, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord. 2010;25(1):97-107. [101] RAY RS, KATYAL A. Myeloperoxidase: bridging the gap in neurodegeneration. Neurosci Biobehav Rev. 2016;68:611-620. [102] UBHI K, INGLIS C, MANTE M, et al. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy. Exp Neurol. 2012;234(2):405-416. [103] METZ LM, LI DKB, TRABOULSEE AL, et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med. 2017;376(22):2122-2133. [104] STEFANOVA N. Translational therapies for multiple system atrophy: bottlenecks and future directions. Auton Neurosci. 2018;211:7-14. [105] YANG HM, YANG S, HUANG SS, et al. Microglial activation in the pathogenesis of Huntington’s disease. Front Aging Neurosci. 2017;9:193. [106] TABRIZI SJ, LEAVITT BR, LANDWEHRMEYER GB, et al. Targeting huntingtin expression in patients with Huntington’s disease. N Engl J Med. 2019;380(24):2307-2316. [107] FEIGIN A, EVANS EE, FISHER TL, et al. Pepinemab antibody blockade of SEMA4D in early Huntington’s disease: a randomized, placebo-controlled, phase 2 trial. Nat Med. 2024;30(2):606. [108] TABRIZI SJ, LEAVITT BR, LANDWEHRMEYER GB, et al. Targeting huntingtin expression in patients with Huntington’s disease. N Engl J Med. 2019;380(24):2307-2316. [109] LI W, QIU J, LI XL, et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci Adv. 2021;7(1):eabd6889. [110] GASCA-SALAS C, FERNÁNDEZ-RODRÍGUEZ B, PINEDA-PARDO JA, et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat Commun. 2021;12(1):779. [111] PROFACI CP, HARVEY SS, BAJC K, et al. Microglia are not necessary for maintenance of blood-brain barrier properties in health, but PLX5622 alters brain endothelial cholesterol metabolism. Neuron. 2024;112(17):2910-2921.e7. [112] YAO X, LYU P, YOO K, et al. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J Extracell Vesicles. 2021;10(5):e12076. [113] SERRANO C, CANANZI S, SHEN T, et al. Simple and highly specific targeting of resident microglia with adeno-associated virus. Preprint. bioRxiv. 2023; 2023.12.12.571321. |
| [1] | 刘 欢, 曾少鹏, 陈 珺, 贺琳茜, 杨 迎, 章 京. 衰老相关的葡萄糖代谢失调:癌症和神经退行性疾病的十字路口[J]. 中国组织工程研究, 2026, 30(6): 1527-1538. |
| [2] | 阴勇成, 赵相瑞, 杨志杰, 李 政, 李 芳, 宁 斌. 过氧化物还原酶1在脊髓损伤后小胶质细胞炎症反应中的作用及机制[J]. 中国组织工程研究, 2026, 30(5): 1106-1113. |
| [3] | 冷晓轩, 赵玉欣, 刘西花. 不同神经调控刺激方式改善帕金森病患者非运动症状的网状Meta分析[J]. 中国组织工程研究, 2026, 30(5): 1282-1293. |
| [4] | 李婷文, 张建华. 水中运动干预老年帕金森病患者平衡功能与运动能力的Meta分析[J]. 中国组织工程研究, 2026, 30(10): 2560-2568. |
| [5] | 迟文鑫, 张存鑫, 高 凯, 吕超亮, 张科峰. 川陈皮素抑制BV2小胶质细胞炎症反应的机制[J]. 中国组织工程研究, 2025, 29(7): 1321-1327. |
| [6] | 何龙才, 宋文学, 明 江, 陈光唐, 王军浩, 廖益东, 崔君拴, 徐卡娅. SD大鼠乳鼠原代皮质神经元和小胶质细胞同时提取并培养的实验方法[J]. 中国组织工程研究, 2025, 29(7): 1395-1400. |
| [7] | 赵瑞华, 陈思娴, 郭 杨, 石 磊, 吴承杰, 吴 毛, 杨光露, 张昊恒, 马 勇. 温肾通督方促进小鼠脊髓损伤的修复[J]. 中国组织工程研究, 2025, 29(6): 1118-1126. |
| [8] | 逯冉冉, 周 旭, 张利杰, 杨新玲. 富马酸二甲酯减轻帕金森病模型鼠神经损伤的作用机制[J]. 中国组织工程研究, 2025, 29(5): 989-994. |
| [9] | 于 辉, 杨 阳, 韦 婷, 李文丽, 罗文倩, 刘 彬. Gadd45b调控星形胶质细胞表型减轻慢性缺血性大鼠脑白质损伤[J]. 中国组织工程研究, 2025, 29(36): 7797-7803. |
| [10] | 郑伊桐, 汪永新, 刘 文, 阿木吉特, 秦 虎. 神经内镜下人脐带间充质干细胞外泌体鞘内移植修复脊髓损伤的作用机制[J]. 中国组织工程研究, 2025, 29(36): 7743-7751. |
| [11] | 水 晶, 何 宇, 江 楠, 徐 坤, 宋丽娟, 丁智斌, 马存根, 李新毅. 星形胶质细胞调节中枢神经系统的髓鞘再生[J]. 中国组织工程研究, 2025, 29(36): 7889-7897. |
| [12] | 徐 彪, 董玉珍, 路 坦. 二氢槲皮素对脊髓损伤大鼠炎症反应标志物表达的影响[J]. 中国组织工程研究, 2025, 29(32): 6843-6850. |
| [13] | 赵 楠, 丁 勇, 修 航, 刘鹏飞, 梁国刚. C57BL/6新生乳鼠肠神经胶质细胞的提取与培养[J]. 中国组织工程研究, 2025, 29(31): 6656-6660. |
| [14] | 汪兆艳, 王 倩, 刘卫鹏, 杨 辉, 栾 佐, 屈素清. 纤维连接蛋白对人神经干细胞诱导分化为少突胶质前体细胞的影响[J]. 中国组织工程研究, 2025, 29(31): 6661-6666. |
| [15] | 张 鑫, 郭宝娟, 徐慧鑫, 沈玉珍, 杨晓帆, 杨旭芳, 陈 培 . 丁苯酞对帕金森病细胞模型的保护作用及机制[J]. 中国组织工程研究, 2025, 29(30): 6466-6473. |
胶质细胞一般被认为是神经元的“支持细胞”,主要起到支持引导神经元迁移、隔离中枢神经分区以及修复损伤神经元等作用。最新研究揭示胶质细胞也可通过代谢物调控[12]、神经突触调控和神经免疫调节等机制[13-14],主动参与神经环路的动态平衡维系中。随着单细胞测序、类器官模型和
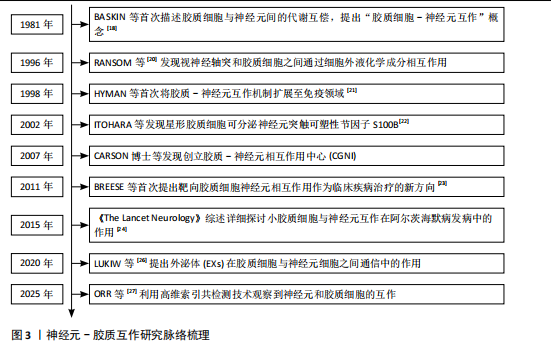
基底节退行性疾病是一类以基底神经节内神经细胞进行性退化和功能障碍为特征的神经系统疾病。端脑内部基底节区是中枢神经系统运动控制和认知调节的核心区域之一[1],主要包括纹状体、黑质、苍白球以及丘脑底核等[2],当该部位发生病变或者神经环路异常时,会表现为如运动迟缓、静止性震颤、肌张力增高等运动功能障碍,以及认知功能或情绪异常等非运动型功能障碍等多种神经退行性疾病[3-4],如帕金森病、亨廷顿病和多系统萎缩等。帕金森病是最常见的基底节退行性疾病,其主要的病理变化是黑质致密部的多巴胺能神经元受累,导致投射至纹状体多巴胺水平下降,从而诱发运动迟缓、震颤、肌强直等运动症状[5]。亨廷顿病是一种遗传相关神经退行性疾病,目前已知由亨廷顿蛋白突变引起,导致纹状体中等棘神经元的进行性丢失[6]。而多系统萎缩则是发病率较低的神经退行性疾病,以α-突触核蛋白和促微管蛋白聚合蛋白在少突胶质细胞中的异常聚集为特征,主要累及基底节、小脑和自主神经系统[7-8]。尽管几种疾病的临床表现和病理特征各不相同,但其特征性病理均以基底节区神经元功能障碍及进行性丢失为主。传统研究主要聚焦于神经元自身异常在疾病发生发展中的作用。但近期研究表明,神经胶质细胞与神经元的相互作用在神经退行性疾病的病理进展中也具有关键作用[9-11],见图1。
胶质细胞一般被认为是神经元的“支持细胞”,主要起到支持引导神经元迁移、隔离中枢神经分区以及修复损伤神经元等作用。最新研究揭示胶质细胞也可通过代谢物调控[12]、神经突触调控和神经免疫调节等机制[13-14],主动参与神经环路的动态平衡维系中。随着单细胞测序、类器官模型和基因编辑技术的快速发展,胶质-神经元互作在基底节退行性疾病中的作用机制逐渐被揭示[15-16]。这些实验结果为深入阐释基底节退行性疾病的病理机制提供了进一步的拓展,但该领域的研究仍面临诸多挑战:首先,胶质细胞在不同疾病阶段的功能转变机制尚不明确,如有报道小胶质细胞可通过香豆素衍生物抑制NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor family pyrin domain containing 3,NLRP3)炎症小体,从促炎M1表型向抗炎M2表型的转变,因而具有神经保护作用[17];然而,小胶质细胞表型转换的具体分子机制及其在疾病不同阶段的动态变化还不清楚;此外,尽管靶向胶质细胞的治疗策略在动物模型中显示出一些潜力,但其临床转化仍面临诸多障碍。
此文综述了胶质-神经元互作在基底节退行性疾病中的研究进展,重点探讨星形胶质细胞、小胶质细胞和少突胶质细胞在帕金森病、亨廷顿病和多系统萎缩中的动态调控机制,同时总结了靶向胶质细胞的治疗策略及其临床应用前景。通过梳理胶质-神经元互作的核心机制和未来研究方向,系统整合领域内最新研究成果,推动相关研究的深入发展,并为基底节退行性疾病的防治提供新的理论依据和治疗思路。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者于2024年6-12月进行文献检索和收集。
1.1.2 检索文献时限 检索2020年1月至2024年11月发表的相关文献(除经典文献外)。
1.1.3 检索数据库 英文数据库为PubMed和Web of Science,中文数据库为中国知网(CNKI)。
1.1.4 英文检索词 基底节相关术语:basal ganglia,striatum,substantia nigra,globus pallidus;胶质细胞相关术语:glial cells,astrocytes,microglia,oligodendrocytes;神经元相关术语:neurons,neurodegeneration,synaptic dysfunction;疾病相关术语:Parkinson’s disease,Huntington’s disease,multiple system atrophy,neurodegenerative diseases;机制相关术语:metabolic coupling,neuroinflammation,synaptic pruning,oxidative stress,organoid model,single-cell sequencing;干预相关术语:therapeutic targets,glial modulation,neuroprotection,blood-brain barrier,off-target effect。
1.1.5 中文检索词 基底节相关术语:基底节,纹状体,黑质,苍白球;胶质细胞相关术语:胶质细胞,星形胶质细胞,小胶质细胞,少突胶质细胞;神经元相关术语:神经元,神经退行性变,突触功能障碍;疾病相关术语:帕金森病,亨廷顿病,多系统萎缩,神经退行性疾病;机制相关术语:代谢耦合,神经炎症,突触修剪,氧化应激,类器官模型,单细胞测序;干预相关术语:治疗靶点,胶质细胞调控,神经保护,血脑屏障,脱靶效应。
1.1.6 检索类型 研究论著、综述和学位论文。
1.1.7 检索策略 PubMed、Web of Science和中国知网数据库检索策略见表1。
1.2 筛选标准
1.2.1 纳入标准 ①研究涉及胶质细胞与神经元互作及动态调节机制,在基底节退行性疾病发病中作用的相关文献;②星形胶质细胞、小胶质细胞、少突胶质细胞在帕金森病、亨廷顿病和多系统萎缩中作用机制研究的相关文献;③靶向星形胶质细胞、小胶质细胞、少突胶质细胞的治疗策略及其在神经退行性病变临床应用的相关文献;④靶向治疗的问题与挑战;⑤单细胞空间转录组在胶质亚型分析中的应用。
1.2.2 排除标准 ①讲座、会议摘要;②非中国科学引文数据库来源的中文文献;③重复研究或数据不完整的文献。
1.3 筛选流程与文献质量评价 共检索到583篇文献,通过阅读标题和摘要对文献进行初步筛选,排除相同研究共232篇,通过阅读全文排除不符合纳入标准的中英文文献共275篇(其中与主题不符268篇,无法获取全文2篇,讲座、会议摘要5篇)。查阅全文后,最终保留76篇文献进行归纳总结。文献筛选流程见图2。
3.1 既往他人在该领域研究的贡献和存在的问题 胶质-神经元互作研究在过去几十年中取得了显著进展。学者们揭示了胶质细胞在神经系统中的重要作用,从最初的“支持细胞”到现在被认为是调控神经环路的关键参与者。早期研究通过蛋白分离技术发现了胶质细胞增生与神经元疾病之间的生化相关性,奠定了胶质-神经元互作的基础。随后,研究者逐步揭示了胶质细胞通过代谢产物调节、免疫互作以及突触修剪等多种机制与神经元相互作用。近年来,随着单细胞测序和类器官模型等技术的发展,胶质-神经元互作在基底节退行性疾病中的分子机制逐渐被揭示。然而,尽管取得了诸多进展,仍存在一些问题:首先,胶质-神经元互作的复杂性和多样性尚未完全阐明,尤其是在不同神经退行性疾病中的动态变化;再者现有研究多集中于单一细胞类型或单一信号通路,缺乏对整体复杂神经网络的系统性研究。许多研究结果停留在动物模型上,其在临床试验中的效果还需进一步证实。
3.2 该综述区别于他人他篇的特点 此综述的特点在于对该领域文献的系统性整合,梳理了胶质-神经元互作的研究进展,整合了其在帕金森病、多系统萎缩和亨廷顿病等代表性基底节退行性疾病中的具体作用机制。从多维度进行分析,涵盖代谢、免疫和突触修剪等多个维度,分析了胶质-神经元互作的复杂性,并探讨了其在疾病进展中的“双刃剑”作用。另外此文对近些年的前沿技术在该领域的应用也进行了探讨,结合单细胞测序、类器官模型和高维空间成像等前沿技术,揭示了胶质-神经元互作在疾病中的分子机制,为未来研究提供了新的视角。最后,此文针对靶向靶向胶质-神经元互作的治疗策略进行了重点探讨,对已有的动物模型以及临床试验进行了汇总归纳,为临床转化提供了理论依据。
3.3 综述的局限性 尽管此综述力求全面,但仍存在一些局限性:首先,研究范围窄而深入,主要聚焦于基底节退行性疾病,未能涵盖胶质-神经元互作在其他神经系统疾病(如自闭症谱系障碍和抑郁症)中的作用;其次,由于该领域的前沿性,大部分汇总的结果都来自于动物模型及细胞实验,考虑到动物模型及细胞系与人类疾病的差异,其在临床中的适用性仍需进一步验证;最后,由于胶质-神经元互作涉及多种细胞类型和信号通路,其机制复杂且多样,此文未能完全涵盖所有可能的相互作用机制。
3.4 综述的重要意义 系统性地总结了胶质-神经元互作的研究进展,为理解基底节退行性疾病的发病机制提供了新的视角;通过探讨胶质-神经元互作在疾病中的作用机制,为开发靶向治疗策略提供了理论依据;同时,结合单细胞测序、类器官模型和高维空间成像等前沿技术,揭示了胶质-神经元互作的分子机制,为未来研究提供了新的技术手段和研究方向。文章强调了胶质细胞与神经元之间关系的复杂性和关键作用,以及胶质细胞在疾病发生发展中的作用。理解这些神经生物学机制在不同疾病病理过程中的作用有助于筛选共同的靶点和创新治疗方法,从而促进基底节退行性疾病机制研究和治疗的发展。
3.5 课题专家组对未来的建议 基于现有研究进展和局限性,课题组专家对未来的研究方向提出以下建议:进一步揭示胶质-神经元互作的分子机制,尤其是在不同疾病状态下的动态变化;结合前沿技术,深入研究胶质-神经元互作的复杂性;推动基础研究成果向临床应用的转化,开展大规模临床试验验证靶向胶质-神经元互作的治疗策略;加强神经科学、免疫学、药理学和临床医学等领域的跨学科合作,推动研究的多学科融合发展。总之,胶质-神经元互作研究为理解基底节退行性疾病的发病机制和开发新型治疗策略提供了重要依据。未来研究应进一步揭示其分子机制,推动临床转化,并加强跨学科合作,为患者带来更多的治疗希望。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||