[1] SKINNIDER MA, GAUTIER M, TEO AYY, et al. Single-cell and spatial atlases of spinal cord injury in the Tabulae Paralytica. Nature. 2024; 631(8019):150-163.
[2] LORACH H, GALVEZ A, SPAGNOLO V, et al. Walking naturally after spinal cord injury using a brain-spine interface. Nature. 2023;618(7963):126-133.
[3] XU L, ZHAO H, YANG Y, et al. The application of stem cell sheets for neuronal regeneration after spinal cord injury: a systematic review of pre-clinical studies. Syst Rev. 2023;12(1):225.
[4] QIU W, ZHOU B, LUO Y, et al. An Optimized Decellularized Extracellular Matrix from Dental Pulp Stem Cell Sheets Promotes Axonal Regeneration by Multiple Modes in Spinal Cord Injury Rats. Adv Healthc Mater. 2024:e2402312.
[5] XU L, YANG Y, ZHONG W, et al. Comparative efficacy of five most common traditional Chinese medicine monomers for promoting recovery of motor function in rats with blunt spinal cord injury: a network meta-analysis. Front Neurol. 2023;14:1165076.
[6] 许卢春,杨永栋,赵赫,等.非编码RNA调控脊髓损伤后神经元细胞凋亡的作用和机制[J].中国组织工程研究,2023,27(33):5404-5412.
[7] JAFFER H, ANDRABI SS, PETRO M, et al. Catalytic antioxidant nanoparticles mitigate secondary injury progression and promote functional recovery in spinal cord injury model. J Control Release. 2023;364:109-123.
[8] ORTEGA MA, FRAILE-MARTINEZ O, GARCÍA-MONTERO C, et al. A comprehensive look at the psychoneuroimmunoendocrinology of spinal cord injury and its progression: mechanisms and clinical opportunities. Mil Med Res. 2023;10(1):26.
[9] RYAN F, BLEX C, NGO TD, et al. Ferroptosis inhibitor improves outcome after early and delayed treatment in mild spinal cord injury. Acta Neuropathol. 2024;147(1):106.
[10] SHI T, CHEN Y, ZHOU L, et al. Carboxymethyl cellulose/quaternized chitosan hydrogel loaded with polydopamine nanoparticles promotes spinal cord injury recovery by anti-ferroptosis and M1/M2 polarization modulation. Int J Biol Macromol. 2024;275(1):133484.
[11] ZHENG Q, WANG D, LIN R, et al. Pyroptosis, ferroptosis, and autophagy in spinal cord injury: regulatory mechanisms and therapeutic targets. Neural Regen Res. 2025;20(10):2787-2806.
[12] HU X, ZHANG H, ZHANG Q, et al. Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J Neuroinflammation. 2022;19(1):242.
[13] LI QS, JIA YJ. Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen Res. 2023;18(3):506-512.
[14] LAN T, SUN TT, WEI C, et al. Epigenetic Regulation of Ferroptosis in Central Nervous System Diseases. Mol Neurobiol. 2023;60(7):3584-3599.
[15] BAI XY, LIU XL, DENG ZZ, et al. Ferroptosis is a new therapeutic target for spinal cord injury. Front Neurosci. 2023;17:1136143.
[16] LIU Y, WAN Y, JIANG Y, et al. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim Biophys Acta Rev Cancer. 2023;1878(3):188890.
[17] ZHANG W, LIU Y, LIAO Y, et al. GPX4, ferroptosis, and diseases. Biomed Pharmacother. 2024;174:116512.
[18] MA T, DU J, ZHANG Y, et al. GPX4-independent ferroptosis-a new strategy in disease’s therapy. Cell Death Discov. 2022;8(1):434.
[19] DING K, LIU C, LI L, et al. Acyl-CoA synthase ACSL4: an essential target in ferroptosis and fatty acid metabolism. Chin Med J (Engl). 2023; 136(21):2521-2537.
[20] JIA B, LI J, SONG Y, et al. ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries. Int J Mol Sci. 2023;24(12):10021.
[21] LIU G, DENG B, HUO L, et al. Tetramethylpyrazine alleviates ferroptosis and promotes functional recovery in spinal cord injury by regulating GPX4/ACSL4. Eur J Pharmacol. 2024;977:176710.
[22] 许卢春,杨永栋,赵赫,等.颈髓损伤动物模型的研究进展[J].中国脊柱脊髓杂志,2023,33(9):836-842.
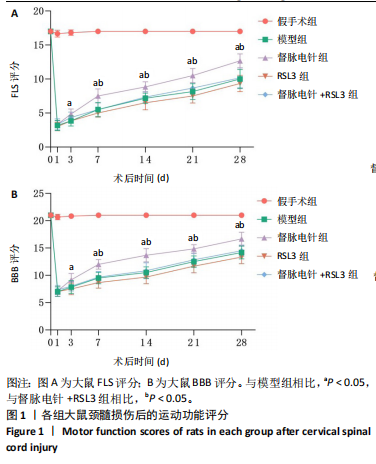
[23] WEBB AA, MUIR GD. Sensorimotor behaviour following incomplete cervical spinal cord injury in the rat. Behav Brain Res. 2005;165(2):147-159.
[24] 罗联忠,周逸敏,张俐.基于督脉枢机不利探讨脊髓损伤与血脊髓屏障[J].中华中医药杂志,2021,36(8):4515-4518.
[25] 吴明莉,冯晓东.基于督脉理论探讨“通督益髓”针法治疗脊髓损伤的研究进展[J].中国民族民间医药,2024,33(14):72-75.
[26] 赵亚莉,姚海江,李志刚,等.督脉电针对脊髓损伤大鼠脊髓神经连接蛋白1和神经丝蛋白200的影响[J].针刺研究,2023,48(9):906-913.
[27] 叶青,李志刚,时素华,等.督脉电针联合重复经颅磁刺激对脊髓损伤后大鼠脊髓BDNF及其受体TrkB和p75NTR表达的影响[J].针灸临床杂志,2022,38(8):57-65.
[28] XU L, YANG Y, JIANG G, et al. Buyang Huanwu decoction promotes angiogenesis and improves hemorheological parameters after cervical spinal cord injury. J Tradit Chin Med Sci. 2024;11(4):456-465.
[29] KANG Y, ZHU R, LI S, et al. Erythropoietin inhibits ferroptosis and ameliorates neurological function after spinal cord injury. Neural Regen Res. 2023;18(4):881-888.
[30] SINGH A, KRISA L, FREDERICK KL, et al. Forelimb locomotor rating scale for behavioral assessment of recovery after unilateral cervical spinal cord injury in rats. J Neurosci Methods. 2014;226:124-131.
[31] BASSO DM, BEATTIE MS, BRESNAHAN JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1-21.
[32] ZIPSER CM, CRAGG JJ, GUEST JD, et al. Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol. 2022;21(7):659-670.
[33] MORITZ C, FIELD-FOTE EC, TEFERTILLER C, et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial. Nat Med. 2024;30(5):1276-1283.
[34] PENG H, LIU Y, XIAO F, et al. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front Bioeng Biotechnol. 2023;11:1111882.
[35] ZHU J, LIU Z, LIU Q, et al. Enhanced neural recovery and reduction of secondary damage in spinal cord injury through modulation of oxidative stress and neural response. Sci Rep. 2024;14(1):19042.
[36] VERSTAPPEN K, AQUARIUS R, KLYMOV A, et al. Systematic Evaluation of Spinal Cord Injury Animal Models in the Field of Biomaterials. Tissue Eng Part B Rev. 2022;28(6):1169-1179.
[37] HU M, CAO Z, JIANG D. The Effect of miRNA-Modified Exosomes in Animal Models of Spinal Cord Injury: A meta-Analysis. Front Bioeng Biotechnol. 2022;9:819651.
[38] KO CC, LEE PH, LEE JS, et al. Spinal decompression surgery may alleviate vasopressor-induced spinal hemorrhage and extravasation during acute cervical spinal cord injury in rats. Spine J. 2024;24(3):519-533.
[39] PIECZONKA K, NAKASHIMA H, NAGOSHI N, et al. Human Spinal Oligodendrogenic Neural Progenitor Cells Enhance Pathophysiological Outcomes and Functional Recovery in a Clinically Relevant Cervical Spinal Cord Injury Rat Model. Stem Cells Transl Med. 2023;12(9):603-616.
[40] YAO S, PANG M, WANG Y, et al. Mesenchymal stem cell attenuates spinal cord injury by inhibiting mitochondrial quality control-associated neuronal ferroptosis. Redox Biol. 2023;67:102871.
[41] XUAN Y, PENG K, ZHU R, et al. Hmox1 is Identified as a Ferroptosis Hub Gene and Associated with the M1 Type Microglia/Macrophage Polarization in Spinal Cord Injury: Bioinformatics and Experimental Validation. Mol Neurobiol. 2023;60(12):7151-7165.
[42] BAO J, YANG S. ScRNA analysis and ferroptosis-related ceRNA regulatory network investigation in microglia cells at different time points after spinal cord injury. J Orthop Surg Res. 2023;18(1):701.
[43] SONG QF, CUI Q, WANG YS, et al. Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury. Neural Regen Res. 2023;18(9):1861-1868.
[44] DIXON SJ, OLZMANN JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25(6):424-442.
[45] JIANG X, STOCKWELL BR, CONRAD M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282.
[46] KIM JW, LEE JY, OH M, et al. An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis. Exp Mol Med. 2023;55(8): 1620-1631.
[47] CUI C, YANG F, LI Q. Post-Translational Modification of GPX4 is a Promising Target for Treating Ferroptosis-Related Diseases. Front Mol Biosci. 2022;9:901565.
[48] DAR NJ, JOHN U, BANO N, et al. Oxytosis/Ferroptosis in Neurodegeneration: the Underlying Role of Master Regulator Glutathione Peroxidase 4 (GPX4). Mol Neurobiol. 2024;61(3):1507-1526.
[49] HUSSAIN S, GUPTA G, SHAHWAN M, et al. Non-coding RNA: A key regulator in the Glutathione-GPX4 pathway of ferroptosis. Noncoding RNA Res. 2024;9(4):1222-1234.
[50] BOUCHAOUI H, MAHONEY-SANCHEZ L, GARÇON G, et al. ACSL4 and the lipoxygenases 15/15B are pivotal for ferroptosis induced by iron and PUFA dyshomeostasis in dopaminergic neurons. Free Radic Biol Med. 2023;195:145-157.
[51] LI R, YAN X, XIAO C, et al. FTO deficiency in older livers exacerbates ferroptosis during ischaemia/reperfusion injury by upregulating ACSL4 and TFRC. Nat Commun. 2024;15(1):4760.
[52] SHI J, XUE X, YUAN L, et al. Amelioration of White Matter Injury Through Mitigating Ferroptosis Following Hepcidin Treatment After Spinal Cord Injury. Mol Neurobiol. 2023;60(6):3365-3378.
[53] DAVAANYAM D, LEE H, SEOL SI, et al. HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain. Exp Mol Med. 2023; 55(11):2402-2416.
[54] LI S, MA S, WANG L, et al. ATF3 as a response factor to regulate Cd-induced reproductive damage by activating the NRF2/HO-1 ferroptosis pathway. Ecotoxicol Environ Saf. 2024;285:117114.
[55] MA H, XING C, WEI H, et al. Berberine attenuates neuronal ferroptosis via the AMPK-NRF2-HO-1-signaling pathway in spinal cord-injured rats. Int Immunopharmacol. 2024;142(Pt B):113227.
[56] RODENCAL J, KIM N, HE A, et al. Sensitization of cancer cells to ferroptosis coincident with cell cycle arrest. Cell Chem Biol. 2024;31(2): 234-248.e13.
[57] JIANG W, YU L, MU N, et al. MG53 inhibits ferroptosis by targeting the p53/SLC7A11/GPX4 pathway to alleviate doxorubicin-induced cardiotoxicity. Free Radic Biol Med. 2024;223:224-236.
[58] LIU N, LIN Q, HUANG Z, et al. Mitochondria-Targeted Prodrug Nanoassemblies for Efficient Ferroptosis-Based Therapy via Devastating Ferroptosis Defense Systems. ACS Nano. 2024;18(11):7945-7958.
[59] WANG LL, MAI YZ, ZHENG MH, et al. A single fluorescent probe to examine the dynamics of mitochondria-lysosome interplay and extracellular vesicle role in ferroptosis. Dev Cell. 2024;59(4):517-528.e3.
[60] ZHONG S, CHEN W, WANG B, et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023;63:102760.
|