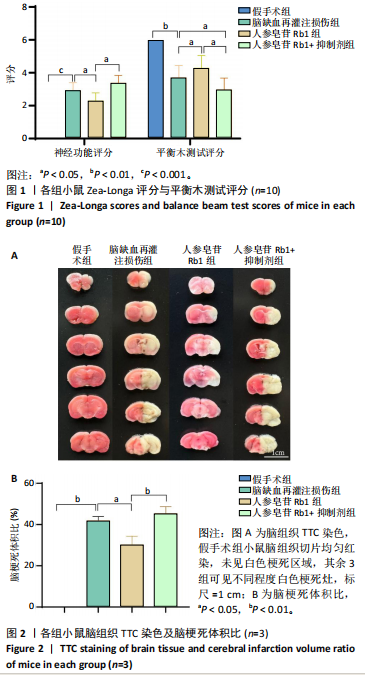
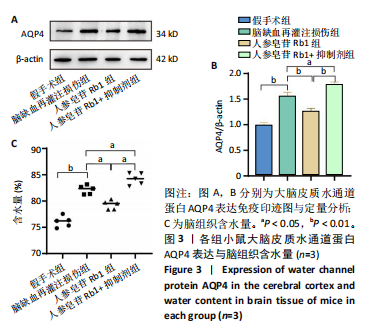
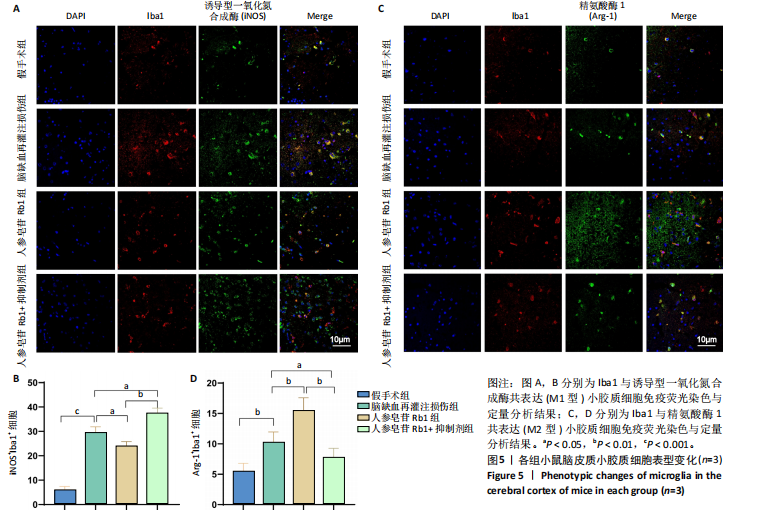
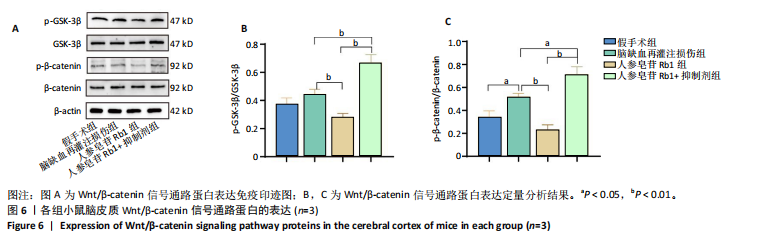
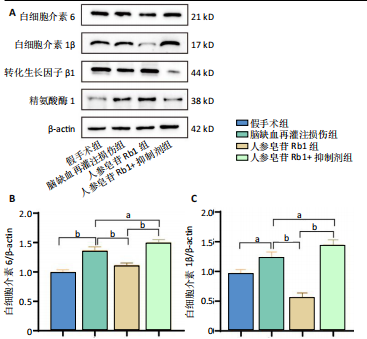
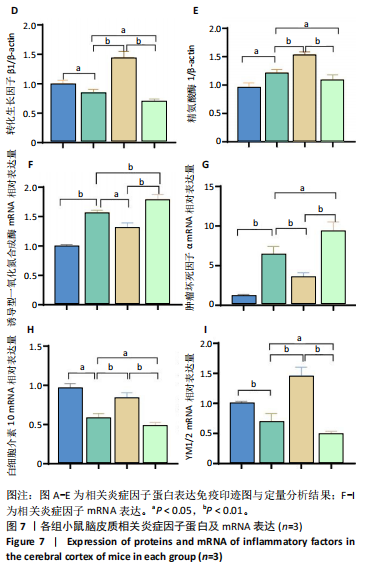
[1] TSAO CW, ADAY AW, ALMARZOOQ ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147(8):e593-e621.
[2] GO AS, MOZAFFARIAN D, ROGER VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399-410.
[3] POWERS WJ, RABINSTEIN AA, ACKERSON T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418.
[4] HERPICH F, RINCON F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48(11):1654-1663.
[5] MAJIDI S, SIMPKINS AN, LEIGH R. The Efficacy of IV Tissue Plasminogen Activator for Restoring Cerebral Blood Flow in the Hours Immediately after Administration in Patients with Acute Stroke. J Neuroimaging. 2019;29(2):206-210.
[6] MOZAFFARIAN D, BENJAMIN EJ, GO AS, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447-454.
[7] DING R, WU W, SUN Z, et al. AMP-activated protein kinase: An attractive therapeutic target for ischemia-reperfusion injury. Eur J Pharmacol. 2020;888:173484.
[8] STOLL G, NIESWANDT B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat Rev Neurol. 2019;15(8):473-481.
[9] ZHANG Q, JIA M, WANG Y, et al. Cell Death Mechanisms in Cerebral Ischemia-Reperfusion Injury. Neurochem Res. 2022;47(12):3525-3542.
[10] LI H, GUO Z, CHEN J, et al. Computational research of Belnacasan and new Caspase-1 inhibitor on cerebral ischemia reperfusion injury. Aging (Albany NY). 2022;14(4):1848-1864.
[11] SONG Z, FENG J, ZHANG Q, et al. Tanshinone IIA Protects Against Cerebral Ischemia Reperfusion Injury by Regulating Microglial Activation and Polarization via NF-κB Pathway. Front Pharmacol. 2021;12:641848.
[12] JURCAU A, SIMION A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int J Mol Sci. 2021;23(1):14.
[13] ARIOZ BI, TASTAN B, TARAKCIOGLU E, et al. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front Immunol. 2019;10:1511.
[14] JOLIVEL V, BICKER F, BINAMÉ F, et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129(2):279-295.
[15] LUO L, LIU M, FAN Y, et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. 2022;19(1):141.
[16] GAO T, HUANG F, WANG W, et al. Interleukin-10 genetically modified clinical-grade mesenchymal stromal cells markedly reinforced functional recovery after spinal cord injury via directing alternative activation of macrophages. Cell Mol Biol Lett. 2022;27(1):27.
[17] ZHANG Z, XU J, ZHANG X, et al. Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits. Toxins (Basel). 2022; 14(9):628.
[18] 赵晓华,田倩倩,薛伟,等.吴茱萸碱通过GSK-3β/β-catenin通路减轻大鼠脑缺血再灌注损伤[J]. 南开大学学报(自然科学版), 2023,56(5):23-28.
[19] SONG D, ZHANG X, CHEN J, et al. Wnt canonical pathway activator TWS119 drives microglial anti-inflammatory activation and facilitates neurological recovery following experimental stroke. J Neuroinflammation. 2019;16(1):256.
[20] JIANG N, ZHANG Y, YAO C, et al. Ginsenosides Rb1 Attenuates Chronic Social Defeat Stress-Induced Depressive Behavior via Regulation of SIRT1-NLRP3/Nrf2 Pathways. Front Nutr. 2022;9:868833.
[21] NI XC, WANG HF, CAI YY, et al. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022;54:102363.
[22] 周璐,陈珊,赵雪,等.人参皂苷Rb1改善局灶性CIRI小鼠模型神经损伤的调控机制研究[J].安徽医科大学学报,2023,58(2):252-258.
[23] BELAYEV L, ALONSO OF, BUSTO R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616-1622; discussion 1623.
[24] ZHAO B, ZHU J, FEI Y, et al. JLX001 attenuates blood-brain barrier dysfunction in MCAO/R rats via activating the Wnt/β-catenin signaling pathway. Life Sci. 2020;260:118221.
[25] ZHOU S, YU G, CHI L, et al. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013;38:136-145.
[26] LIU K, LI L, LIU Z, et al. Acute Administration of Metformin Protects Against Neuronal Apoptosis Induced by Cerebral Ischemia-Reperfusion Injury via Regulation of the AMPK/CREB/BDNF Pathway. Front Pharmacol. 2022;13:832611.
[27] ZHENG Y, HU Y, HAN Z, et al. Lomitapide ameliorates middle cerebral artery occlusion-induced cerebral ischemia/reperfusion injury by promoting neuronal autophagy and inhibiting microglial migration. CNS Neurosci Ther. 2022;28(12):2183-2194.
[28] ZHANG Z, SONG Z, SHEN F, et al. Ginsenoside Rg1 Prevents PTSD-Like Behaviors in Mice Through Promoting Synaptic Proteins, Reducing Kir4.1 and TNF-α in the Hippocampus. Mol Neurobiol. 2021;58(4):1550-1563.
[29] WEN S, ZOU ZR, CHENG S, et al. Ginsenoside Rb1 improves energy metabolism after spinal cord injury. Neural Regen Res. 2023;18(6): 1332-1338.
[30] ZHOU T, ZU G, ZHANG X, et al. Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology. 2016;101: 480-489.
[31] CHEN H, FENG Z, MIN L, et al. Vagus Nerve Stimulation Reduces Neuroinflammation Through Microglia Polarization Regulation to Improve Functional Recovery After Spinal Cord Injury. Front Neurosci. 2022;16:813472.
[32] KUMAR A, ALVAREZ-CRODA DM, STOICA BA, et al. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma. 2016;33(19):1732-1750.
[33] AZEEM W, BAKKE RM, APPEL S, et al. Dual Pro- and Anti-Inflammatory Features of Monocyte-Derived Dendritic Cells. Front Immunol. 2020; 11:438.
[34] WU Y, DU J, WU Q, et al. The osteogenesis of Ginsenoside Rb1 incorporated silk/micro-nano hydroxyapatite/sodium alginate composite scaffolds for calvarial defect. Int J Oral Sci. 2022;14(1):10. |