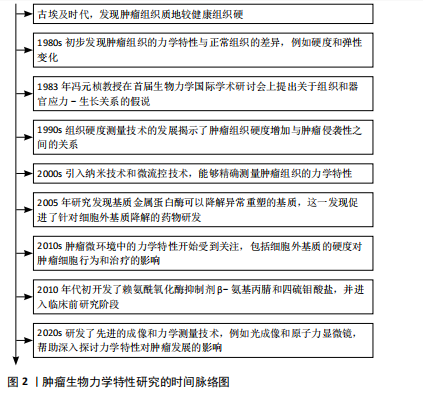
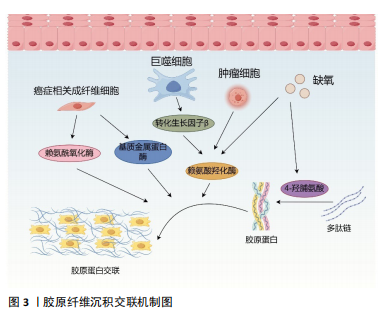
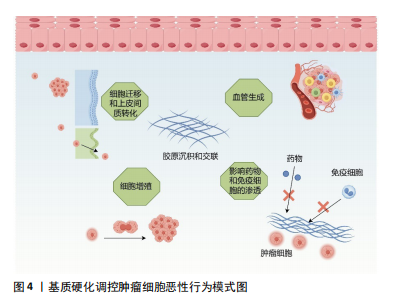
[1] CHEN F, ZHUANG X, LIN L, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45.
[2] KARAMANOS NK, THEOCHARIS AD, PIPERIGKOU Z, et al. A guide to the composition and functions of the extracellular matrix. Febs J. 2021; 288(24):6850-6912.
[3] BONNANS C, CHOU J, WERB Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786-801.
[4] BOYD DF, THOMAS PG. Towards integrating extracellular matrix and immunological pathways. Cytokine. 2017;98:79-86.
[5] WALKER C, MOJARES E, DEL RíO HERNÁNDEZ A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018; 19(10):3028.
[6] NAGELKERKE A, BUSSINK J, ROWAN AE, et al. The mechanical microenvironment in cancer: How physics affects tumours. Semin Cancer Biol. 2015;35:62-70.
[7] WEI J, YAO J, YAN M, et al. The role of matrix stiffness in cancer stromal cell fate and targeting therapeutic strategies. Acta Biomater. 2022;150: 34-47.
[8] NIA HT, MUNN LL, JAIN RK. Physical traits of cancer. Science. 2020; 370(6516):eaaz0868.
[9] JAIN RK, MARTIN JD, STYLIANOPOULOS T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16: 321-346.
[10] ULRICH TA, DE JUAN PARDO EM, KUMAR S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69(10):4167-4174.
[11] CHEN G, XIA B, FU Q, et al. Matrix Mechanics as Regulatory Factors and Therapeutic Targets in Hepatic Fibrosis. Int J Biol Sci. 2019;15(12): 2509-2521.
[12] JIANG Y, ZHANG H, WANG J, et al. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15(1):34.
[13] XU S, XU H, WANG W, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309.
[14] RØMER AMA, THORSETH ML, MADSEN DH. Immune Modulatory Properties of Collagen in Cancer. Front Immunol. 2021;12:791453.
[15] LIU J, SHEN JX, WU HT, et al. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov Med. 2018;25(139):211-223.
[16] LIANG Y, LV Z, HUANG G, et al. Prognostic significance of abnormal matrix collagen remodeling in colorectal cancer based on histologic and bioinformatics analysis. Oncol Rep. 2020;44(4):1671-1685.
[17] BROOKS M, MO Q, KRASNOW R, et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget. 2016;7(50):82609-82619.
[18] VOILES L, LEWIS DE, HAN L, et al. Overexpression of type VI collagen in neoplastic lung tissues. Oncol Rep. 2014;32(5):1897-1904.
[19] CHEN S, WEI Y, LIU H, et al. Analysis of Collagen type X alpha 1 (COL10A1) expression and prognostic significance in gastric cancer based on bioinformatics. Bioengineered. 2021;12(1):127-137.
[20] HISATOMI K, MUKAE H, SAKAMOTO N, et al. Pirfenidone inhibits TGF-β1-induced over-expression of collagen type I and heat shock protein 47 in A549 cells. BMC Pulm Med. 2012;12:24.
[21] GHOSH D, MEJIA PENA C, QUACH N, et al. Senescent mesenchymal stem cells remodel extracellular matrix driving breast cancer cells to a more-invasive phenotype. J Cell Sci. 2020;133(2):jcs232470.
[22] PANKOVA D, JIANG Y, CHATZIFRANGKESKOU M, et al. RASSF1A controls tissue stiffness and cancer stem-like cells in lung adenocarcinoma. Embo J. 2019;38(13):e100532.
[23] NECULA L, MATEI L, DRAGU D, et al. Collagen Family as Promising Biomarkers and Therapeutic Targets in Cancer. Int J Mol Sci. 2022; 23(20):12415.
[24] GILKES DM, BAJPAI S, CHATURVEDI P, et al. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819-10829.
[25] CABRAL-PACHECO GA, GARZA-VELOZ I, CASTRUITA-DE LA ROSA C, et al. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. J Mol Sci. 2020;21(24):9739.
[26] ACERBI I, CASSEREAU L, DEAN I, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb). 2015;7(10):1120-1134.
[27] LACHOWSKI D, CORTES E, RICE A, et al. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep. 2019;9(1):7299.
[28] RAMOS S, FERREIRA S, FERNANDES AS, et al. Lysyl Oxidases Expression and Breast Cancer Progression: A Bioinformatic Analysis. Front Pharmacol. 2022;13:883998.
[29] ZHU J, LUO C, ZHAO J, et al. Expression of LOX Suggests Poor Prognosis in Gastric Cancer. Front Med (Lausanne). 2021;8:718986.
[30] KIM YM, KIM EC, KIM Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38(1):145-149.
[31] SCHMELZER CEH, HEINZ A, TROILO H, et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. Faseb J. 2019;33(4):5468-5481.
[32] 唐晓男, 康小红. 赖氨酸羟化酶2在肿瘤转移中的研究进展[J]. 医学研究生学报,2019,32(10):1099-1103.
[33] ISHIHARA S, HAGA H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers (Basel). 2022;14(4):1049.
[34] DENG B, ZHAO Z, KONG W, et al. Biological role of matrix stiffness in tumor growth and treatment. J Transl Med. 2022;20(1):540.
[35] LIU C, PEI H, TAN F. Matrix Stiffness and Colorectal Cancer. Onco Targets Ther. 2020;13:2747-2755.
[36] CALVO F, EGE N, GRANDE-GARCIA A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013; 15(6):637-646.
[37] PIERSMA B, HAYWARD MK, WEAVER VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356.
[38] GENG X, CHEN H, ZHAO L, et al. Cancer-Associated Fibroblast (CAF) Heterogeneity and Targeting Therapy of CAFs in Pancreatic Cancer. Front Cell Dev Biol. 2021;9:655152.
[39] BATES ME, LIBRING S, REINHART-KING CA. Forces exerted and transduced by cancer-associated fibroblasts during cancer progression. Biol Cell. 2023;115(8):e2200104.
[40] LI X, SUN Z, PENG G, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022; 12(2):620-638.
[41] SCHRADER J, GORDON-WALKER TT, AUCOTT RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192-1205.
[42] 刘秋萍, 田博仁, 罗庆, 等. 基质刚度对肝癌细胞增殖及糖代谢的影响[J]. 医用生物力学,2019,34(2):133-138.
[43] KHALIL K, EON A, JANODY F. Cell Architecture-Dependent Constraints: Critical Safeguards to Carcinogenesis. Int J Mol Sci. 2022;23(15):8622.
[44] PROVENZANO PP, KEELY PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124(Pt 8):1195-1205.
[45] PANZETTA V, VERDE G, PUGLIESE M, et al. Adhesion and Migration Response to Radiation Therapy of Mammary Epithelial and Adenocarcinoma Cells Interacting with Different Stiffness Substrates. Cancers (Basel). 2020;12(5):1170.
[46] 彭悦婷. 基质刚度通过ROCK及NMII亚型差异调控乳腺癌细胞迁移的机制研究[D]. 成都: 电子科技大学,2021.
[47] WU S, ZHENG Q, XING X, et al. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J Exp Clin Cancer Res. 2018;37(1):99.
[48] LAMOUILLE S, XU J, DERYNCK R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178-196.
[49] XU J, LAMOUILLE S, DERYNCK R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156-172.
[50] DONG Y, ZHENG Q, WANG Z, et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol. 2019;12(1):112.
[51] WEI SC, FATTET L, TSAI JH, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17(5):678-688.
[52] XU X, ZHANG Y, WANG X, et al. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int J Mol Sci. 2021;22(21):12066.
[53] YANG L, LI J, ZANG G, et al. Pin1/YAP pathway mediates matrix stiffness-induced epithelial-mesenchymal transition driving cervical cancer metastasis via a non-Hippo mechanism. Bioeng Transl Med. 2023;8(1): e10375.
[54] DENG Y, CHAKRABORTY P, JOLLY MK, et al. A Theoretical Approach to Coupling the Epithelial-Mesenchymal Transition (EMT) to Extracellular Matrix (ECM) Stiffness via LOXL2. Cancers (Basel). 2021;13(7):1609.
[55] TAO B, SONG Y, WU Y, et al. Matrix stiffness promotes glioma cell stemness by activating BCL9L/Wnt/β-catenin signaling. Aging (Albany NY). 2021;13(4):5284-5296.
[56] SAFAEI S, SAJED R, SHARIFTABRIZI A, et al. Tumor matrix stiffness provides fertile soil for cancer stem cells. Cancer Cell Int. 2023;23(1): 143.
[57] PANG M F, SIEDLIK MJ, HAN S, et al. Tissue Stiffness and Hypoxia Modulate the Integrin-Linked Kinase ILK to Control Breast Cancer Stem-like Cells. Cancer Res. 2016;76(18):5277-5287.
[58] TIAN B, LUO Q, JU Y, et al. A Soft Matrix Enhances the Cancer Stem Cell Phenotype of HCC Cells. Int J Mol Sci. 2019;20(11):2831.
[59] 陈瑜, 白红霞, 彭悦婷, 等. 胞外基质刚度对骨肉瘤干细胞自我更新的调控机制研究[J]. 医用生物力学,2019,34(S1):66-67.
[60] BERTERO T, OLDHAM WM, GRASSET EM, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019;29(1):124-140.e110.
[61] GE H, TIAN M, PEI Q, et al. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front Oncol. 2021;11:631991.
[62] KALLI M, POSKUS MD, STYLIANOPOULOS T, et al. Beyond matrix stiffness: targeting force-induced cancer drug resistance. Trends Cancer. 2023;9(11):937-954.
[63] TRÉDAN O, GALMARINI CM, PATEL K, et al. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441-1454.
[64] QIN X, LV X, LI P, et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165625.
[65] LI M, ZHANG X, WANG M, et al. Activation of Piezo1 contributes to matrix stiffness-induced angiogenesis in hepatocellular carcinoma. Cancer Commun (Lond). 2022;42(11):1162-1184.
[66] CARMELIET P, JAIN RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298-307.
[67] WANG Y, ZHANG X, WANG W, et al. Integrin αVβ5/Akt/Sp1 pathway participates in matrix stiffness-mediated effects on VEGFR2 upregulation in vascular endothelial cells. Am J Cancer Res. 2020; 10(8):2635-2648.
[68] GUO Y, MEI F, HUANG Y, et al. Matrix stiffness modulates tip cell formation through the p-PXN-Rac1-YAP signaling axis. Bioact Mater. 2022;7:364-376.
[69] JIANG X, HU J, WU Z, et al. Protein Phosphatase 2A Mediates YAP Activation in Endothelial Cells Upon VEGF Stimulation and Matrix Stiffness. Front Cell Dev Biol. 2021;9:675562.
[70] KRETSCHMER M, RüDIGER D, ZAHLER S. Mechanical Aspects of Angiogenesis. Cancers (Basel). 2021;13(19):4987.
[71] LV B, WANG Y, MA D, et al. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front Immunol. 2022;13:844142.
[72] CHEN Y, ZHAO M, ZHANG L, et al. SNF5, a core subunit of SWI/SNF complex, regulates melanoma cancer cell growth, metastasis, and immune escape in response to matrix stiffness. Transl Oncol. 2022;17:101335.
[73] MAO X, XU J, WANG W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131.
[74] EDWARDS DR, MURPHY G, REYNOLDS JJ, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. Embo j. 1987;6(7):1899-1904.
[75] LIU YL, BAGER CL, WILLUMSEN N, et al. Tetrathiomolybdate (TM)-associated copper depletion influences collagen remodeling and immune response in the pre-metastatic niche of breast cancer. NPJ Breast Cancer. 2021;7(1):108.
[76] SCHILTER H, FINDLAY AD, PERRYMAN L, et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J Cell Mol Med. 2019;23(3):1759-1770.
[77] KANEDA A, SEIKE T, DANJO T, et al. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am J Cancer Res. 2020;10(12):4399-4415.
[78] LI X, SONG Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13(1): 50.
[79] MAI Z, LIN Y, LIN P, et al. Modulating extracellular matrix stiffness: a strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024;15(5):307.
[80] JOHNSON LA, MORGAN RA, DUDLEY ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3): 535-546.
|