中国组织工程研究 ›› 2023, Vol. 27 ›› Issue (17): 2669-2674.doi: 10.12307/2023.186
• 组织构建细胞学实验 cytology experiments in tissue construction • 上一篇
少突胶质前体细胞延长神经胶质母细胞瘤大鼠高剂量放疗后的生存期
高 月1,蔺建文1,李 迪1,蓝晓艳1,李 深2,储成艳1
- 1大连医科大学附属大连市中心医院,辽宁省大连市 116033;2首都医科大学附属北京世纪坛医院,北京市 100038
Oligodendrocyte progenitor cells prolong the survival of glioblastoma-bearing rats after high-dose radiotherapy
Gao Yue1, Lin Jianwen1, Li Di1, Lan Xiaoyan1, Li Shen2, Chu Chengyan1
- 1Dalian Municipal Central Hospital, Dalian Medical University, Dalian 116033, Liaoning Province, China; 2Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China
摘要:
文题释义:
放疗性脑损伤:放疗是治疗神经胶质瘤的一线治疗手段。然而,辐射可造成少突胶质细胞丢失等健康脑组织的损伤,进而引起一系列不良反应,目前尚缺乏有效的修复手段。
少突胶质前体细胞:作为重要的祖细胞库,少突胶质前体细胞可快速分化为成熟的少突胶质细胞,替代损伤、死亡的少突胶质细胞以维持中枢神经系统髓鞘结构及功能的稳定。
背景:胶质母细胞瘤是成人常见的恶性脑肿瘤,目前尚缺乏有效的治疗方法,临床预后不良。
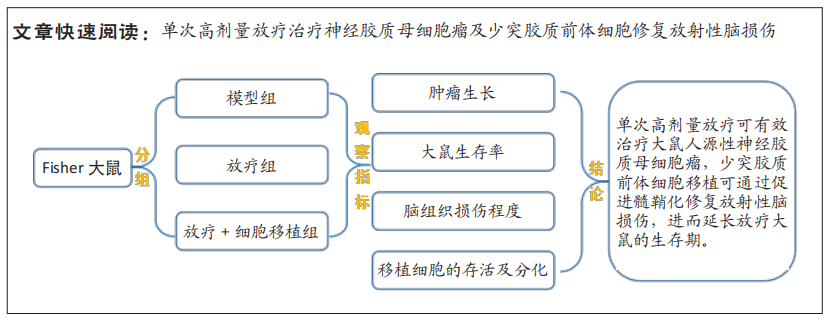
目的:观察单次高剂量放疗对神经胶质母细胞瘤的疗效,以及少突胶质前体细胞修复放射性脑损伤的可行性。
方法:通过质粒转染构建可表达荧光素酶(Luc)的人源性U-87 MG肿瘤细胞系。15只Fisher 344大鼠制备Luc-U-87 MG神经胶质母细胞瘤模型,随机分为肿瘤模型组(n=3)、放疗组(n=6)和放疗+细胞移植组(n=6),后两组放疗方式为单次80 Gy辐射;放疗后5周,放疗+细胞移植组大鼠联合少突胶质前体细胞移植治疗。利用活体成像的方法监测肿瘤细胞的生长;MRI观察放疗引起的脑组织影像学变化;采用Kaplan-Meier生存分析明确细胞移植对放疗大鼠生存率的影响;对移植细胞的存活及分化情况进行组织学观察。
结果与结论:①体外生物成像示转染后的U-87 MG细胞与Luc底物产生反应发出生物信号,信号强度与转染细胞的数量呈线性正相关;②动物活体成像结果显示,肿瘤模型大鼠的颅内胶质母细胞瘤信号持续上升直至接种第5周(全部死亡);放疗大鼠在治疗后2周,肿瘤信号开始衰减,4周后未见肿瘤发光信号;③放疗后5周,T2WI上可见放疗大鼠坏死的肿瘤组织;10周后未见肿瘤显影,但出现脑组织损伤信号,少突胶质前体细胞移植大鼠脑组织未见类似信号;15周后,放疗组和放疗+细胞移植组大鼠在T2WI上均可见高、低混杂的组织损伤信号,但放疗+细胞移植组的损伤信号程度明显低于单纯放疗组;④生存分析结果显示模型组、放疗组及放疗+细胞移植组大鼠的中位生存期分别为30 d,114.5 d和232.5 d,各组大鼠的中位生存期之间差异均有显著性意义(P < 0.01);⑤组织学观察到存活的移植细胞,呈多极样,部分细胞表达髓鞘碱性蛋白,且放疗+细胞移植组的髓鞘碱性蛋白表达明显高于放疗组(P < 0.01);⑥上述结果表明,单次高剂量放疗可有效治疗大鼠人源性神经胶质母细胞瘤,少突胶质前体细胞移植可通过修复放射性脑损伤延长放疗大鼠的生存期,促进髓鞘化是其修复损伤脑组织的机制之一。
https://orcid.org/0000-0003-2916-2882(高月)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: